EAS Consensus Panel paper: Simplifying the definition of hypertriglyceridaemia
Molecular genetic analyses have facilitated dramatic progress in understanding the mechanisms potentially underlying hypertriglyceridaeridaemic states. Thus, the focus of the latest paper from the European Atherosclerosis Society (EAS) Consensus Panel[1] on hypertriglyceridaemia is timely. The EAS Consensus Panel calls for a simplified dichotomous redefinition of hypertriglyceridaemia, recognising that the majority of cases have a polygenic basis. This simplified definition (see below) has important implications for the diagnosis and management of hypertriglyceridaemia, and is therefore critically relevant to a wide range of clinicians routinely involved in the management of dyslipidaemia.
According to Lead author, Robert A. Hegele, Distinguished Professor of Medicine and Biochemistry, Schulich School of Medicine and Dentistry, Western University, London, Ontario, Canada:
‘Hypertriglyceridemia represents a diagnostic and therapeutic challenge for many health care providers. This consensus paper synthesizes recent epidemiologic, genetic and clinical evidence, streamlining the definition of hypertriglyceridaemia, which in turn serves as a simplified framework for diagnosis and management.’
The full paper is available for download HERE; the pdf will also be available as open access on Lancet Diabetes Endocrinology for February [at http://www.thelancet.com/journals/landia/article/PIIS2213-8587(13)70191-8/fulltext].
Triglycerides and cardiovascular disease
High levels of triglycerides, a marker for triglyceride-rich lipoproteins (TRLs) and their remnants, have a role in cardiovascular disease (CVD). Indeed, a previous EAS Consensus Panel position paper[2] concluded that evidence from mechanistic and genetic studies, as well as epidemiological data supported a causal association of elevated TRLs and their remnants (with or without low high-density lipoprotein [HDL] cholesterol) with elevated CVD risk. Subsequent to that publication, there were supportive findings from a Mendelian randomisation study suggesting that lifelong exposure to remnant TRLs is causal for coronary heart disease risk, independent of low plasma concentrations of HDL cholesterol.[3] Furthermore, a genetic study indicated a causal role for triglycerides, independent of confounding effects due to low-density lipoprotein (LDL) cholesterol or HDL cholesterol in the development of coronary artery disease.[4] However, it is recognised that we still lack definitive evidence that targeting elevated triglycerides impacts CVD outcomes. This because the studies conducted to date have been flawed by inclusion of patients without clinically relevant hypertriglyceridaemia, and therefore, are not a true test of this hypothesis.
Understanding the genetic basis of hypertriglyceridaemia: monogenic versus polygenic
Historically, the phenotypic heterogeneity of hypertriglyceridaemia was defined according to qualitative and quantitative differences in plasma lipoproteins using the Fredrickson classification of hyperlipoproteinaemia phenotypes.[5] It was implicit in this classification that differences between different hypertriglyceridaemic phenotypes were due to genetic differences.
It is recognised that hypertriglyceridaemia tends to cluster in families, implying a genetic component. However, recent genetic insights indicate that in more than 95% of cases of hypertriglyceridaemia, there is a polygenic or multigenic basis explaining this susceptibility.[2,6-10] The phenotypic heterogeneity of hypertriglyceridaemia reflects the cumulative burden associated with common small-effect genetic variants, as well as rare heterozygous large-effect variants of genes directly or indirectly associated with plasma triglycerides (Figure 1). Thus, while most cases of hypertriglyceridaemia are inherited, they are not monogenic.
Figure 1.
As shown, most cases of hypertriglyceridaemia can be considered polygenic or multigenic
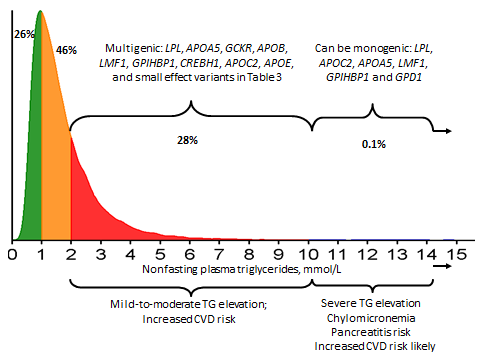
Adapted from Hegele RA et al (2013).[1]
Monogenic hypertriglyceridaemia
Indeed, monogenic hypertriglyceridaemia is rare, thought to affect about one in a million people. Such individuals have markedly elevated triglyceride levels (>10 mmol/L or >885 mg/dL), usually evident from a young age. In many cases, these individuals are homozygous or compound heterozygous (i.e. with two recessive alleles for the same gene, but with those two alleles being different from each other) for large-effect loss of function mutations in 6 different genes that regulate the catabolism of TRLs (i.e. LPL, APOC2, APOA5, LMF1, GP1HBP1 and GPD1).
Polygenic hypertriglyceridaemia
However, most cases of hypertriglyceridaemia are polygenic, that is, the phenotype reflects the cumulative burden of common small-effect and rare large-effect variants of more than 30 genes involved in regulating the production or catabolism (or both) of TRLs. In other words, an increased burden of variants associated with triglyceride- raising translates to increased susceptibility to hypertriglyceridaemia. This substantially complicates tracing the hypertriglyceridaemic phenotype through families.
Moreover, susceptibility to elevated triglycerides is also impacted by secondary causes of hypertriglyceridaemia (see Box 1), which are themselves influenced by a genetic susceptibility component, leading to clustering in families. Notably obesity, metabolic syndrome, non-alcoholic fatty liver disease and diabetes, have their own susceptibility component.
Finally, It should be borne in mind that even among individuals with markedly elevated triglycerides (>10 mmol/L), the underlying genetic basis may be polygenic, compounded by secondary factors, if no monogenic cause is identified.
| Box 1. Secondary causes of hypertriglyceridaemia |
| • Obesity • Metabolic syndrome • Diet (high positive energy intake, high-fat or high simple carbohydrate diets) • Increased alcohol intake (conventionally defined as >2 units daily in men and >1 unit daily in women) • Diabetes (mainly type 2) • Hypothyroidism • Renal disease (proteinuria, uraemia, glomerulonephritis) • Pregnancy (especially in third trimester) • Paraproteinaemia • Systemic lupus erythematosus • Drugs (including corticosteroids, oral oestrogen, tamoxifen, thiazides, non-cardioselective beta-blockers, bile acid sequestrants, cyclophosphamide, asparaginase, protease inhibitors, and second-generation antipsychotics) |
EAS Consensus Panel recommendations for redefinition of hypertriglyceridaemia
Taking account of these new genetic data, the EAS Consensus Panel therefore recommends a simplified redefinition of hypertriglyceridaemia, as either mild to moderate, or severe (Box 2). The Panel took the decision to use a diagnostic cutpoint of 2.0 mmol/L (175 mg/dL). However, it is recognised that this threshold is arbitrary, as consensus indicates that values ±0.3 mmol/L around this cutpoint may be also considered.
| Box 2. Simplified definition of hypertriglyceridaemia |
| • Mild to moderate hypertriglyceridaemia: 2-10 mmol/L (175-885 mg/dL) • A polygenic basis accounts for the phenotypic heterogeneity of triglyceride levels • Routine genetic testing is not recommended • Severe hypertriglyceridaemia: >10 mmol/L (>885 mg/dL) • Likely to have a monogenic basis; however, a polygenic basis cannot be discounted • Other than in children and adolescents, routine genetic testing is not recommended |
Targets
The EAS Consensus Panel recognises that there is inadequate evidence to define specific treatment targets for plasma triglycerides. In spite of this, both the EAS Consensus Panel and the European Society of Cardiology (ESC)/EAS guidelines for management of dyslipidaemia consider a triglyceride concentration <1.7 mmol/L (<150 mg/dL) as desirable, especially in the context of low plasma HDL cholesterol concentration.[2,11] Nonfasting lipid measurement may improve the efficiency of screening and diagnosis of hypertriglyceridaemia.[12]
Non-HDL cholesterol and apolipoprotein B (apoB) (Table 1) are recommended secondary targets in defining desirable levels in individuals at high risk of CVD, consistent with current guidelines.[2,11-13]
| Table 1. Desirable levels for lipids and lipoproteins in individuals at high risk of CVD based on current guidance2,11,13,14 | ||
| Parameter | Represents | Desirable level |
| Triglycerides | Marker of TRLs and remnants | <1.7 mmol/L (<150 mg/dL) |
| Non-HDL cholesterol | Cholesterol in LDL and remnant TRLs, i.e. total mass of cholesterol in atherogenic lipoprotein particles | <2.6 mmol/L (<100 mg/dL) |
| ApoB | Total number of apoB-containing atherogenic lipoprotein particles | <0.8 g/L in high-risk <0.7 g/L in very high-risk |
Managing hypertriglyceridaemia
There are two key aims in the management of hypertriglyceridaemia:
- Immediate prevention of acute pancreatitis in patients with severe hypertriglyceridaemia (>10 mmol/L); fibrates are first-line treatment; and
- Reduction of global CVD risk
After addressing secondary causes (see Box 1), management of hypertriglyceridaemia should be in accordance with current guidelines (see Table 2).[11,13] Patients should be managed for their global CVD risk; a positive family history of CVD (at least one first degree relative or at least 2 second degree relatives with CVD) should be taken into account, independent of dyslipidaemia. As elevated LDL cholesterol is also part of the phenotype of combined hyperlipidaemia which exacerbates CVD risk, family members should be screened.
The primary priority for intervention is LDL cholesterol, with non-HDL cholesterol and apoB secondary treatment targets after attainment of LDL cholesterol goal. Lifestyle intervention is the mainstay of the management of hypertriglyceridaemia (after decreasing pancreatitis risk in patients with severe hypertriglyceridaemia). In terms of pharmacotherapy, statins are justifiably recommended for management of hypertriglyceridaemia due to i) proven efficacy in reducing CVD risk and ii) efficacy in lowering plasma triglycerides by up to 30% depending on baseline levels and dose. In Europe, the addition of a fibrate (especially in individuals with concomitant low plasma levels of HDL cholesterol), or omega-3 fatty acids may be considered, in accordance with current guidance.
| Table 2. Management of hypertriglyceridaemia | ||
| Mild-moderate hypertriglyceridaemia | Severe hypertriglyceridaemia | |
| Overall aim | Prevent CVD | Prevent acute pancreatitis |
| Primary goal | Achieve LDL-C target | Reduce triglycerides |
| Secondary priorities | Achieve non-HDL-C or apoB targets (see Table 1) Assess and treat secondary factors | Achieve LDL-C and non-HDL-C targets after acute pancreatitis risk is decreased |
| Management strategies | Lifestyle intervention: reduce weight, improve diet*, reduce alcohol intake and increase aerobic activity Statin to control LDL-C, titrating dose to achieve LDL-C and non-HDL-C targets; addition of a fibrate, nicotinic acid** or omega-3 fatty acids may be considered if non-HDL-C target is still not met | Manage acute pancreatitis in accordance with current guidance; Lifestyle intervention for the longer-term: strict fat-reduced diet (<20% of calories as fat), reduce bodyweight; reduce intake of alcohol, improve diet*; increase aerobic activity Consider fibrate, nicotinic acid** and omega-3 fatty acids |
* Reduce simple sugar and total carbohydrate intake; replace trans and saturated fat with monounsaturated fats; increase dietary omega-3 fatty acids;
** Nicotinic acid is no longer an option in Europe following the withdrawal of extended release niacin/laropiprant
Looking to the future
Expanding research is focused on elucidating the genetics of different forms of hypertriglyceridaemia, which may help in the identification of therapeutic targets. Ultimately these new research directions may offer the prospect of personalised medicine in the setting of hypertriglyceridaemia.
References
- Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, Borén J, Bruckert E, Catapano AL, Descamps OS, Hovingh GK, Humphries SE, Kovanen PT, Masana L, Pajukanta P, Parhofer KG, Raal FJ, Ray KK, Santos RD, Stalenhoef AFH, Stroes E, Taskinen M-R, Tybjærg-Hansen A, Watts GF, Wiklund O, on behalf of the European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2013. Published Online December 23, 2013. http://dx.doi.org/10.1016/S2213-8587(13)70191-8.
- Chapman MJ, Ginsberg HN, Amarenco P et al; European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345-61
- Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 2013;61:427–36.
- Do R, Willer CJ, Schmidt EM et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013 Oct 6. doi: 10.1038/ng.2795.
- Fredrickson DS, Lees RS. A system for phenotyping hyperlipoproteinemia. Circulation 1965;31:321–27.
- Hegele RA, Ban MR, Hsueh N et al. A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia. Hum Mol Genet 2009;18:4189–94.
- Johansen CT, Wang J, Lanktree MB et al. An increased burden of common and rare lipid-associated risk alleles contributes to the phenotypic spectrum of hypertriglyceridemia. Arterioscler Thromb Vasc Biol 2011;31:1916–26.
- Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J Lipid Res 2011;52:189–206.
- Johansen CT, Hegele RA. Genetic bases of hypertriglyceridemic phenotypes. Curr Opin Lipidol 2011;22:247–53.
- Johansen CT, Hegele RA. Allelic and phenotypic spectrum of plasma triglycerides. Biochim Biophys Acta 2012;1821:833–42.
- Catapano AL, Reiner Z, De Backer G et al, and the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS), and the ESC Committee for Practice Guidelines 2008–2010 and 2010–2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011;217 (suppl 1): S1–44.
- Nordestgaard BG, Freiberg JJ. Clinical relevance of non-fasting and postprandial hypertriglyceridemia and remnant cholesterol. Curr Vasc Pharmacol 2011;9:281–86.
- Anderson TJ, Grégoire J, Hegele RA et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013; 29: 151–67.
- The International Atherosclerosis Society. An International Atherosclerosis Society Position Paper: Global recommendations for the management of dyslipidemia. Full report [http://www.athero.org/download/IASPPGuidelines_FullReport_2.pdf]