EAS Consensus Panel Statement on Homozygous FH
The European Atherosclerosis Society (EAS) Consensus Panel on Familial Hypercholesterolaemia (FH) has published a position statement on homozygous FH. This follows the first EAS Consensus Statement, which highlighted the extent of underdiagnosis and undertreatment of heterozygous FH.[1]
The publication is available to download from:
http://eurheartj.oxfordjournals.org/content/35/32/2146
Why focus on homozygous FH?
Homozygous (HoFH) is a rare and life-threatening disease characterised by markedly elevated circulating levels of low-density lipoprotein cholesterol (LDL-C) and accelerated, premature atherosclerotic cardiovascular disease (ACVD). The first major CV events often occur during adolescence, although angina pectoris, myocardial infarction and death have been reported in early childhood.[2-5] Historically thought to affect one in a million,[6] new research indicates that HoFH prevalence is likely to be higher, with as many as one in 160,000 to 300,000 people affected.[1,7] Thus, rather than ~6,000 there may be as many as 40,000 people world-wide with HoFH.
Due to the severity of the condition, early diagnosis and initiation of lipid-lowering treatment is critical. Given that heterozygous FH is underdiagnosed, there are clearly missed opportunities for diagnosis of HoFH. This issue is further complicated by recent evidence showing that the phenotypic heterogeneity of HoFH is more pronounced than originally believed.
With the approval of two novel treatments, lomitapide and mipomersen, as well as recent data showing the efficacy of the pro-protein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibody evolocumab, there has been renewed interest in HoFH. Consequently, this EAS Consensus Panel statement is timely in providing clinical guidance for the recognition and management of HoFH patients. These recommendations are clearly relevant not only to cardiologists and lipid specialists, but also to a wide spectrum of clinicians, including primary care physicians and paediatricians, who are often the first to see patients with HoFH.
Understanding the genetics of HoFH
HoFH is usually characterised by a lack of LDL receptors, due to the presence of two mutant alleles of the LDLR gene. However, other genes have been identified as causal in some cases with a severe phenotype resembling HoFH; these include APOB (encoding apolipoprotein [apo] B), PCSK9 (encoding PCSK9), and LDLRAP1 (encoding LDLR adapter protein 1, which plays a role in mediating the endocytosis of the LDL receptor-LDL complex during receptor recycling). It is important to note that mutation in LDLRAP1 causes a recessive phenotype, since carrier parents have normal lipid profiles.[8] The frequency of FH-causing mutations is: LDLR (>95%), APOB (2-5%), PCSK9 (<1%) and LDLRAP1 (<1%). There may be other causative genes so far not identified. Overall, the vast majority of HoFH patients are homozygotes, with the same mutation in both alleles of the same gene (usually LDLR), or compound heterozygotes, with different mutations in each allele of the same gene coding for the LDL receptor. Rarely, HoFH patients may have mutations in two different FH-causing genes, one within the LDLR gene and one in another gene, and are referred to as double heterozygotes.
Irrespective of the underlying genetic defect, the severity of the HoFH phenotype depends on residual LDL receptor activity. In patients with a lack of LDL receptor-activity (LDLR-negative, <2% residual activity), clinical severity is greater than among those who are profoundly deficient (LDLR-defective, 2-25% residual activity).[2,9,10] Response to conventional lipid-lowering therapies (see below) may be also limited due to LDL marked or almost absent receptor deficiency.
Recommendations for diagnosis
The EAS Consensus Panel recommendations for the diagnosis of HoFH are summarised in Box 1. Diagnosis of HoFH should be made by careful assessment of the clinical characteristics and family history. Genetic testing may provide a definitive diagnosis of HoFH. However, even with over 1,200 FH-causing mutations identified to date, in a proportion of patients no genetic mutation is detected.
| Box 1. EAS Consensus Panel recommendations for diagnosis of HoFH |
| • Genetic confirmation of two mutant alleles at the LDLR, APOB, PCSK9 or LDLRAP1 gene locus OR • An untreated LDL-C > 13 mmol/L (500 mg/dL) or treated LDL-C ≥ 8 mmol/L (300 mg/dL)* together with either: • Cutaneous or tendon xanthoma before age 10 years or • Untreated elevated LDL-C levels consistent with heterozygous FH in both parents * These LDL-C levels are only indicative, and lower levels, especially in children or in treated patients, do not exclude HoFH. |
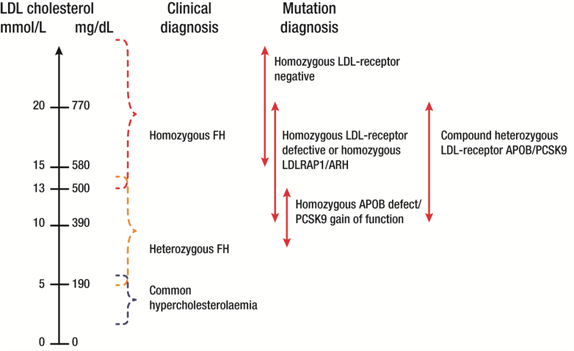
Within the family, the plasma LDL-C level is the critical discriminator, with levels ~4 times higher in family members with HoFH compared with unaffected members.[8] The presence of cutaneous or tuberous xanthomas before age 10 years, as well as arcus corneae, is highly suggestive of diagnosis. At the population level, however, there can be considerable overlap in plasma LDL-C levels between heterozygous and homozygous FH (Figure 1), as well as the age of appearance of xanthomas, largely as a result of the genetic heterogeneity of FH.[1,7,9] It is essential that individuals with suspected HoFH are promptly referred to specialist centres for a comprehensive ACVD evaluation and clinical management.
Figure 1.
Variability in plasma LDL-C levels with FH
Finally, as well as being the ‘gold standard’ for diagnosis of HoFH, genetic testing also has a crucial role in identifying the presence of FH-causing mutations in family members via reverse cascade screening) (Box 2).
| Box 2. Role of genetic testing for FH |
| Genetic analysis should be considered to • Confirm the clinical diagnosis • Facilitate testing of family members (reverse cascade screening) • Assist in diagnosis where clinical presentation is borderline between that of HoFH and heterozygous FH |
Screening for atherosclerotic disease
Accelerated, premature ACVD, due to the burden of markedly elevated plasma LDL-C levels from birth, is the clinical hallmark of HoFH.[1] As ACVD risk is proportional to cholesterol-year score, the higher the cholesterol level and the longer the duration of exposure, the greater the risk of premature coronary artery disease, irrespective of whether a genetic mutation is identified.[11] Untreated, HoFH patients who are LDLR-negative rarely survive beyond 20 years; even those who are LDL-defective usually develop clinically significant ACVD by age 30 years.[2]
The pattern of ACVD typically affects the aortic root, although other territories may be affected. In young children, the first symptoms and signs are frequently linked to aortic stenosis and regurgitation.[6,12]
Given the extremely high risk of early onset of severe ACVD and its rapid progression, regular screening for subclinical aortic and coronary heart disease (CHD) is essential. The EAS Consensus Panel screening recommendations are given in Box 3. The atherosclerotic burden of the aorta can be also evaluated by magnetic resonance imaging (MRI) or trans-oesophageal echocardiography.[13-15] Stress testing, although not optimal for detecting subclinical disease, may be an alternative where there is limited access to computed tomography coronary angiography (CTCA) or cardiac magnetic resonance imaging (MRI).
In patients with clinical symptoms suggestive of ischaemia or valve malfunction, stress testing and invasive coronary angiography are indicated. The latter is recommended in severely affected young children, and should be performed by an experienced paediatric invasive cardiologist. Coronary revascularisation is indicated for severe CHD, and aortic valve replacement for severe left ventricle outflow obstruction. For either surgery, management of the aortic root, which is usually compromised by atherosclerotic plaques and calcification is critical; reconstruction may be necessary with aortic valve replacement.[16]
| Box 3. EAS Consensus Panel recommendations: screening for subclinical ACVD |
| • Patients should undergo comprehensive cardiovascular evaluation at diagnosis, with subsequent Doppler echocardiographic evaluation of the heart and aorta annually, stress testing and, if available, CTCA every 5 years or more frequently if needed. |
Management of HoFH
The EAS Consensus Panel recommendations for management of HoFH are summarised in Box 4. Lifestyle intervention and maximal statin therapy are the mainstays of treatment, and should be started in the first year of life or at initial diagnosis. However, even at the highest doses of the most efficacious statins, only modest reductions in LDL C plasma levels, of 10-25%, are observed. Combination of statin plus ezetimibe can produce a further decrease in LDL-C levels of 10-15%. Combination with other lipid-lowering treatments may be also considered, depending on their availability and tolerability.
The LDL-C targets recommended in the previous EAS Consensus Panel statement related to heterozgous FH were <2.5 mmol/L [<100 mg/dL] (<3.5 mmol/L [<135 mg/dL] in children), or <1.8 mmol/L [<70 mg/dL] in adults with clinical ACVD.[1] However, it is well recognised that these targets are ambitious. While similar targets are recommended for patients with HoFH, these are very rarely achieved with conventional treatments, especially given the phenotypic and genetic heterogeneity of HoFH. Benefit versus risk considerations, especially in the paediatric setting, are clearly relevant.
Lipoprotein apheresis is an important adjunctive treatment in HoFH, which can help in LDL-C goal attainment, with close to normal LDL-C levels achieved with a once weekly regimen.[17-19] Supportive evidence shows that long-term lipoprotein apheresis can contribute to plaque regression and/or stabilisation and improve prognosis,[19] and is cost effective in HoFH.[20,21] Ideally, treatment should be started by age 5 and not later than 8 years, although the Panel recognises that practical feasibility and cost also influence decisions regarding treatment.
Novel therapeutic approaches in HoFH, including the recently licensed treatments lomitapide and mipomersen, as well as PCSK9 monoclonal antibody therapy, offer the potential for further substantial LDL-C lowering (by 25-50%) on top of current standards of care, including maximal statin therapy (Figure 2; see below).[22-24]
Figure 2.
Schematic showing absolute LDL-C lowering with mipomersen, lomitapide and PCSK9 monoclonal antibody therapy.
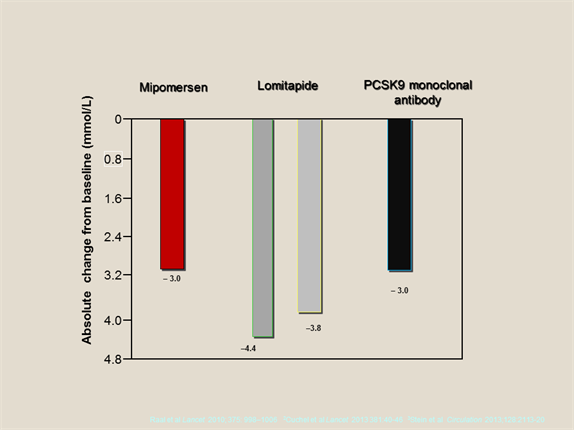
Data from Cuchel et al (2013)[22], Raal et al (2010)[23] and Stein et al (2013)[24]
| Box 4. EAS Consensus Panel recommendations for management of HoFH Recommendations for management |
| • Current management of HoFH focuses on a combination of lifestyle, statin treatment (with or without ezetimibe) and lipoprotein apheresis, if available. • Lipid-lowering therapy should be started as early as possible. • Lipoprotein apheresis should be considered in all patients with HoFH, and started as soon as possible, ideally by age 5 and not later than 8 years. • Lomitapide and mipomersen should be considered as adjunctive treatments to further lower plasma LDL-C levels in patients with HoFH. |
Treatment innovations: are LDL-C targets achievable?
Lomitapide and mipomersen have been recently approved in the US as adjunct therapy for HoFH in patients aged ≥18 and ≥12 years, respectively; lomitapide is also approved in Europe. Both target the production and secretion of apoB-containing lipoproteins, albeit via different mechanisms. Lomitapide, an oral treatment, inhibits microsomal triglyceride transport protein (MTP), which is responsible for loading triglycerides and phospholipids onto nascent chylomicrons in the intestine and very low-density lipoproteins in the liver, resulting in reduced secretion into the circulation.[25] Mipomersen, administered by subcutaneous injection, is a second generation antisense oligonucleotide, which targets the messenger ribonucleic acid of apo B, leading in turn to reduced synthesis of apo B by the ribosome.[26]
In clinical trials,[22,23] both agents have shown efficacy in reducing plasma LDL-C levels by (25-50%) in patients with HoFH, on top of the standard of care, including apheresis, with reduction in LDL-C levels dependent on baseline LDL-C levels. In the case of lomitapide, the reduction in plasma LDL-C levels (by ~50%) was durable over the long-term (>12 months). There are, however, recognised side effect issues, including gastrointestinal symptoms (lomitapide), injection site reactions (mipomersen) and liver enzyme elevation and liver fat accumulation (both agents).[22,23,27] This profile of adverse effects highlights the need for a proper risk/benefit evaluation when considering initiation of these treatments.
Additionally, results from the TESLA study, reported at the first latebreaker session at EAS Congress Madrid,[28] indicate a role for the PCSK9 monoclonal antibody evolocumab in HoFH patients. This Phase III randomised, placebo-controlled trial was conducted in HoFH patients (92% with LDLR mutations) who were not adequately treated with conventional treatment (intensive statin therapy with ezetimibe, mean LDL-C 9.0 mmol/L). Overall, treatment with evolocumab was associated with 30% reduction in LDL-C levels in patients with LDLR mutations, and was well tolerated. Efficacy was greater in LDL-deficient patients (i.e. two defective LDL alleles, -31.8% versus +15.2% with placebo, treatment difference -46.9%, p<0.001) compared with LDL-defective patients (i.e. one defective allele, -21.0% versus +3.5%, treatment difference -24.5%, p<0.013). Other approaches under investigation in the setting of HoFH include inhibitors of cholesteryl ester transfer protein, as well as gene replacement therapy.[29,30]
Beyond management of hypercholesterolaemia, HoFH patients and their families should be educated about the condition and offered psychological support as integral components of patient care. In female patients, there are also issues concerning contraception, and where not contraindicated, pregnancy (Box 5).
| Box 5. Other management issues to consider in the patient with HoFH |
| • Hormonal contraception is generally contraindicated in HoFH, and other contraceptive methods are strongly preferred. Women wishing to become pregnant should be counselled and undergo detailed CV assessment. Where pregnancy is not contraindicated, women should remain on LDL apheresis. • Psychological support should be integrated into routine care. • Surgery may be considered to remove large cutaneous or tuberous xanthomas for either functional or cosmetic reasons. |
The future looks promising for the management of HoFH. Novel therapies, two of which are currently licensed, offer potential for achieving LCL-C goal and improving prognosis in these difficult to treat patients. The EAS believes that this timely Consensus Statement, together with current and future therapeutic innovations, may help to raise awareness and improve clinical outcome for patients with this rare, life-threatening genetic disease.
References
- Nordestgaard BG, Chapman MJ, Humphries SE et al; European Atherosclerosis Society Consensus Panel. Eur Heart J 2013; Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478-90.
- Kolansky DM, Cuchel M, Clark BJ et al. Longitudinal evaluation and assessment of cardiovascular disease in patients with homozygous familial hypercholesterolemia. Am J Cardiol 2008;102:1438-43.
- Macchiaiolo M, Gagliardi MG, Toscano A, Guccione P, Bartuli A. Homozygous familial hypercholesterolaemia. Lancet 2012;379:133.
- Raal FJ, Pilcher GJ, Panz VR et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation 2011;124:2202-7.
- Al-Shaikh AM, Abdullah MH, Barclay A et al. Impact of the characteristics of patients and their clinical management on outcomes in children with homozygous familial hypercholesterolemia. Cardiol Young 2002;12:105-112
- Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolaemia. In: Scriver CR, Beaudet AL, Sly WS, Vale D, eds. In: The Metabolic and Molecular Basis of Inherited Disease. 8th ed., vol. III. New York: McGraw Hill; 2001. p2863–2914.
- Sjouke B, Kusters DM, Kindt I et al. Homozygous autosomal dominant hypercholesterolemia in the Netherlands: prevalence, genotype-phenotype relationship and clinical outcome. Eur Heart J 2014; Feb 28. [Epub ahead of print]
- Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med 2007;4:214-25.
- Moorjani S, Roy M, Torres A et al. Mutations of low-density-lipoprotein-receptor gene, variation in plasma cholesterol, and expression of coronary heart disease in homozygous familial hypercholesterolaemia. Lancet 1993;341:1303-6.
- Bertolini S, Pisciotta L, Rabacchi C et al. Spectrum of mutations and phenotypic expression in patients with autosomal dominant hypercholesterolemia identified in Italy. Atherosclerosis 2013;227:342-8.
- Schmidt HH, Hill S, Makariou EV et al. Relation of cholesterol-year score to severity of calcific atherosclerosis and tissue deposition in homozygous familial hypercholesterolemia. Am J Cardiol 1996;77:575-80.
- Raal FJ, Santos RD. Homozygous familial hypercholesterolemia: Current perspectives on diagnosis and treatment. Atherosclerosis 2012;223:262-8.
- Santos RD, Miname MH, Martinez LR et al. Non-invasive detection of aortic and coronary atherosclerosis in homozygous familial hypercholesterolemia by 64 slice multi-detector row computed tomography angiography. Atheroslerosis 2008;197:910-5.
- Summers RM, Andrasko-Bourgeois J et al. Evaluation of the aortic root by MRI: insights from patients with homozygous familial hypercholesterolemia. Circulation 1998;98:509-18.
- Koh TW. Aortic root involvement in homozygous familial hypercholesterolemia–transesophageal echocardiographic appearances of supravalvular aortic stenosis. Echocardiography 2005;22:859-60.
- Grenon SM, Lachapelle K, Marcil M, Omeroglu A, Genest J, de Varennes B. Surgical strategies for severe calcification of the aorta (porcelain aorta) in two patients with homozygous familial hypercholesterolemia. Can J Cardiol 2007;23:1159-61.
- Thompson GR, Barbir M, Davies D et al. Recommendations for the use of LDL apheresis. Atherosclerosis 2008: 98:247-55.
- Stefanutti C, Julius U. Lipoprotein apheresis: state of the art and novelties. Atheroscler Suppl 2013;14:19-27.
- Schuff-Werner P, Fenger S, Kohlschein P. Role of lipid apheresis in changing times. Clin Res Cardiol Suppl 2012;7(Suppl 1):7-14.
- Watts GF, Gidding S, Wierzbicki AS et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol 2014;171:309-25.
- Health Quality Ontario. Low-density lipoprotein apheresis: an evidence-based analysis. Ont Health Technol Assess Ser 2007;7:1-101.
- Cuchel M, Meagher EA, du Toit Theron H et al; Phase 3 HoFH Lomitapide Study investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 2013;381:40-6.
- Raal FJ, Santos RD, Blom DJ et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:998-1006.
- Stein EA, Honarpour N, Wasserman SM et al. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation 2013;128:2113-20.
- Hussain MM, Rava P, Walsh M et al. Multiple functions of microsomal triglyceride transfer protein. Nutr Metab (Lond) 2012;9:14.
- Crooke ST, Geary RS. Clinical pharmacological properties of mipomersen (Kynamro), a second generation antisense inhibitor of apolipoprotein B. Br J Clin Pharmacol 2013;76:269-76.
- Stein EA, Dufour R, Gagne C et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 2012;126:2283-92.
- Raal F, Honarpour N, Blom DJ et al. Trial evaluating evolocumab, a PCSK9 antibody in patients with homozygous FH (TESLA): results of the randomized, double-blind placebo-controlled trial. Atherosclerosis 2014;235:e12 [abstract].
- Raal FJ, Marais AD, Gagne C et al. Torcetrapib/atorvastatin substantially raises HDL-C and markedly lowers LDL-C in patients with homozygous familial hypercholesterolemia. Atherosclerosis Suppl 2007;8:204 (Abstract P023-764).
- Grossman M, Rader DJ, Muller DW et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med 1995;1:1148-54.