New EAS Consensus Statement on FH: Improving the care of FH patients
This month’s commentary focuses on familial hypercholesterolaemia (FH), a common genetic dyslipidaemia. If left untreated, FH markedly increases the risk of coronary heart disease (CHD); at age 60 years, this risk is at least 50% in men and 30% in women.1 However, if diagnosed and appropriately treated, patients with heterozygous FH can have a normal life expectancy.
This scenario highlights two crucial needs: to diagnose FH early and treat FH effectively. These have been the drivers for the most recent Consensus Statement of the European Atherosclerosis Society (EAS),2 available to download at http://eurheartj.oxfordjournals.org/content/early/2013/08/15/eurheartj.eht273.short?rss=1.
The EAS believes that this timely Consensus Statement will make a real difference to FH patients and save lives.
Scope of the problem: FH is underdiagnosed
Before the publication of this Consensus Statement, it was generally accepted that about one in 500 people in the general population have heterozygous FH, and one in a million have homozygous FH.3 However, these values have been questioned as they were derived from clinical data from over 30 years ago, using less accurate methods.
Indeed, recent data from the Copenhagen General Population Study, an unselected North European general population sample, highlight that these values markedly underestimate the prevalence of FH. Based on the Dutch Lipid Clinic Network criteria for FH (regarded as the gold standard), it was estimated that about one in 200 people had definite or probable FH.4 These estimates therefore suggest that up to 34 million people may be affected by FH worldwide (Figure 1).
Figure 1.
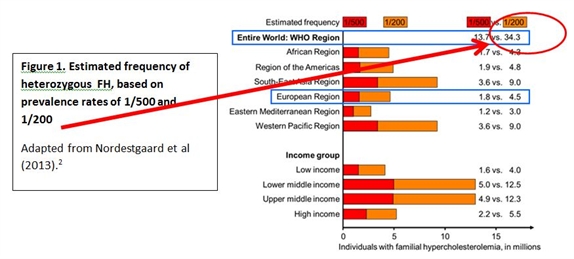
However, it is clear that with few exceptions, the efficiency in diagnosing FH lags far behind. In most countries, less than 1% of FH patients are diagnosed.2 The reasons for this are multiple, and include the lack of valid nationwide registries for FH; lack of screening strategies to detect index cases; and cost issues likely to impact access to genetic testing. Importantly, there is a lack of awareness of FH at the primary care. Clearly, there is a need for urgent action to educate and improve diagnosis of FH.
Need to do better: EAS Consensus Panel recommendations
Addressing this issue, the EAS Consensus Panel has had made a number of recommendations to improve screening for FH (Table 1).2
| Table 1. EAS Consensus Panel Recommendations: Who to screen for FH |
| The EAS Consensus Panel recommends that children, adults and families should be screened for FH if the following apply: • A family member has FH For individuals or family members: • For adults, plasma cholesterol >8.0 mmol/L (>310 mg/dL)* • For children, plasma cholesterol >6.0 mmol/L (>230 mg/dL)* • Premature CHD • Tendon xanthomas • Sudden premature coronary death in a family member (<55 years in men and <60 years in women) *or >95th percentile by age and gender for country in the individual or family member |
The EAS Consensus Panel recommends cascade screening of family members of known FH cases based on measurement of plasma levels of low-density lipoprotein (LDL) cholesterol as the most cost-effective screening method for identifying new FH subjects. Index cases can be identified by opportunistic or targeted screening in primary care or in hospital settings. Ideally, screening should be systematic, centrally co-ordinated by a specialised centre and based on both plasma lipid profiles and genetic testing. However, if genetic testing is not available, this can be based on the plasma lipid profile alone.
The Panel recommends the use of the Dutch Lipid Clinic Network Criteria in adults to establish the clinical diagnosis of FH in adults (Table 2). In individuals with definite/probable FH (score >5), genetic testing is recommended.
The Dutch Lipid Clinic Network Criteria are not appropriate in children. For children, the optimal age for screening is between 2 and 10 years. Evidence of hypercholesterolaemia (total or LDL cholesterol) on repeat testing after 2-3 months on dietary intervention, should be a driver for further testing. In children with a parent with FH, an LDL cholesterol >3.5 mmol/L (>135 mg/dL) is strongly suggestive of FH. Genetic testing is recommended in all children of FH parents.
Genetic testing is now available in many countries for mutations in the LDLR, APOB, PCSK9 and LDLRAP genes that are causative for FH.5 However, the EAS Consensus Panel recognises that despite improved understanding the genetic causes of FH, no causal mutation is detected in a proportion (10-40%) of patients with clinical FH.6,7 It may be that other key genes are implicated, or that there are multiple small-effect genes (i.e. polygenic causality) that contribute to increased LDL cholesterol. Irrespective of a genetic diagnosis, all subjects with a clinical diagnosis of FH (see Table 2) should be treated with an effective LDL cholesterol lowering agent.
For both adults and children, it is also important that secondary causes of hypercholesterolaemia are excluded by checking liver, renal and thyroid function and confirming that there is no evidence of hyperglycaemia or albuminuria.
| Table 2. Dutch Lipid Clinic Network criteria for diagnosis of FH in adults | |
| Group 1 Family history | Points |
| First degree relative with • Known premature CHD (<55 years in men, <60 years in women) OR | 1 |
| • Known LDL cholesterol >95th percentile by age and gender for country • Tendon xanthoma and/or corneal arcus OR • Children <18 years with LDL cholesterol >95th percentile by age and gender for country | 1 2 2 |
| Group 2 Clinical history | |
| Subject with • Premature CHD (as defined above) • Premature cerebral or peripheral vascular disease (as defined above) | 2 1 |
| Group 3 Clinical examination | |
| • Tendon xanthoma • Corneal arcus in a person <45 years | 6 4 |
| Group 4 Biochemistry (LDL cholesterol) | |
| • >8.5 mmol/L (>325 mg/dL) • 6.5-8.5 mmol/L (251-325 mg/dL) • 5.0-6.4 mmol/L (191-250 mg/dL) • 4.0-4.9 mmol/L (155-190 mg/dL) | 8 5 3 1 |
| Group 5 Molecular genetic testing | |
| Causative mutation in the LDLR, APOB, or PCSK9 genes | 8 |
| The highest single score in each group is considered. Score >8 definite FH; 6-8 probable FH; 3-5 possible FH; 0-2 unlikely FH | |
FH is undertreated
As highlighted by the EAS Consensus Panel, the severity of atherosclerosis and coronary disease in FH is proportional to the cumulative burden of elevated LDL cholesterol l levels. Studies have shown that an LDL cholesterol burden sufficient to cause CHD develops at least 20 years earlier in untreated FH patients. For example, the cumulative LDL burden at age 35 years in untreated patients with heterozygous FH is similar to that in a 55-year old individual without FH.2,8,9 However, if effective treatment is instituted from an early age and LDL cholesterol targets are met (see Table 3), this burden can be substantially reduced.
These data clearly highlight the critical importance of effective treatment of FH to reduce the LDL cholesterol burden and risk for atherosclerosis and CHD.
| Table 3. Recommended LDL cholesterol targets for FH patients: EAS Consensus Panel and Joint ESC/EAS guidelines2,10 |
| • Children: <3.5 mmol/L (<135 mg/dL) • Adults: <2.5 mmol/L (<100 mg/dL) • Adults with CHD or diabetes <1.8 mmol/L (<70 mg/dL) Targets are the same in heterozygous and homozygous FH |
However, undertreatment of FH is a major issue. Less than <5% of patients achieve the recommended LDL cholesterol targets. In suboptimally treated patients with FH, coronary risk may be up to 13-fold higher than in those who attain recommended LDL cholesterol targets.4
Need to do better: EAS Consensus Panel recommendations
The EAS Consensus Panel recommends that patients with FH should be initiated on maximal doses of potent statins at first consultation (Table 4).2,10 However, as it is likely that many of these patients will not attain LDL cholesterol targets on statin monotherapy, additional therapy will be required.
The EAS Consensus Panel2 recommends the use of statin-ezetimibe combination therapy in these patients, which is effective in reducing LDL cholesterol levels by 60-70%. For very high risk FH patients with CHD or diabetes, the addition of a bile acid-binding resin is recommended. Addition of a fibrate (fenofibrate) to maximal statin dose may be considered in FH patients with elevated triglycerides and low HDL cholesterol.2,11
| Table 4. |
| Children • Consider starting treatment at 8-10 years, in addition to lifestyle management • Treatment priorities: statin, ezetimibe, bile acid-binding resin. Only those statins shown to be safe in children should be prescribed. • Lipoprotein apheresis in children with homozygous FH |
| Adults • Start treatment on diagnosis, in addition to lifestyle management • Treatment priorities: maximal potent statin dose*, ezetimibe, bile acid-binding resin • Lipoprotein apheresis in homozygous FH and treatment-resistant heterozygous FH with CHD *Maximal potent statin dose could be either atorvastatin 80 mg, rosuvastatin 40 mg, or pitavastatin 4 mg. Simvastatin 80 mg should not be used due to the potential increased risk of myopathy. |
The EAS Consensus Panel recommends that most patients with heterozygous FH be managed in primary care, preferably in a family context. A ‘shared care’ approach between primary care and specialised lipid or FH clinics may also be useful.
However, the Panel recognises that some FH patients will require specialist management, notably i) patients with statin intolerance; ii) patients with CHD at very high cardiovascular risk and iii) patients with very high LDL cholesterol levels despite treatment (including those with homozygous FH). The latter two patient categories may be considered for adjunctive lipoprotein apheresis at specialised centres.12
New focus on FH: novel therapies
FH has attracted renewed focus with the development of novel therapies that are effective in lowering LDL cholesterol by 60% or more. Such agents include:
- Therapeutic strategies targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), including monoclonal antibody therapies. Other treatments including siRNA, vaccines and small molecule therapeutics targeting PCSK9 are at an earlier stage of development.
- Antisense oligonucleotides targeting apolipoprotein B (such as mipomersen) and
- Microsomal triglyceride transfer protein inhibitors (such as lomitapide).
The recent approval of mipomersen and lomitapide as adjunctive therapy in the setting of homozygous FH has been regarded as a ‘new dawn’ for this serious, rare condition.
However, the impact of the EAS Consensus Panel Recommendations and advent of novel therapies targeting LDL will be greatest in the very much larger group of patients with heterozygous FH.
The EAS believes that this Consensus Statement on FH will act as a driver to raise awareness of FH in primary care, where earlier diagnosis and effective treatment can markedly improve life expectancy among FH patients.
References
- Slack J. Risks of ischaemic heart disease in familial hyperlipoproteinaemic states. Lancet 1969;2:1380-2
- Nordestgaard BG, Chapman MJ, Humphries SE et al; European Atherosclerosis Society Consensus Panel. Eur Heart J 2013; Epub ahead of print August 15, 2013.
- Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolaemia. In: Scriver CR, Beaudet AL, Sly WS, Vale D, eds. In: The Metabolic and Molecular Basis of Inherited Disease. 8th ed., vol. III. New York: McGraw Hill; 2001. p2863–2914.
- Benn M,Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab 2012;97:3956–3964.
- Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. J Clin Invest 2003;111:1795-1803.
- Civeira F, Ros E, Jarauta E et al. Comparison of genetic versus clinical diagnosis in familial hypercholesterolemia. Am J Cardiol 2008;102:1187–1193.
- Palacios L, Grandoso L, Cuevas N et al. Molecular characterization of familial hypercholesterolemia in Spain. Atherosclerosis 2012;221:137–142.
- Huijgen R, Hutten BA, Kindt I, Vissers MN, Kastelein JJ. Discriminative ability of LDL-cholesterol to identify patients with familial hypercholesterolemia: a crosssectional study in 26,406 individuals tested for genetic FH. Circ Cardiovasc Genet 2012;5:354–359.
- Starr B, Hadfield SG, Hutten BA et al. Development of sensitive and specific age- and gender-specific low-density lipoprotein cholesterol cutoffs for diagnosis of first-degree relatives with familial hypercholesterolaemia in cascade testing. Clin Chem Lab Med 2008;46:791–803.
- Catapano AL, Reiner Z, De Backer G et al. ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011;217:3-46.
- Robinson JG, Goldberg AC. Treatment of adults with familial hypercholesterolemia and evidence for treatment: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011;5:S18–S29.
- Schuff-Werner P, Fenger S, Kohlschein P. Role of lipid apheresis in changing times. Clin Res Cardiol Suppl 2012;7:7–14