Atherosclerosis newsletter
Highlighted articles from March issue Vol 390
Newsletter by Editor in Chief Prof Arnold von Eckardstein, and Editorial Office Manager Simona Negrini
Join the Atherosclerosis social media community on Facebook (Atherosclerosis – Journal of the European Atherosclerosis Society) and Twitter (@ATHjournal) for scientific discussion and information about the journal.
Inflammation plays an important role in the pathogenesis of atherosclerotic cardiovascular diseases (ASCVD) and is hence targeted by both diagnostics and therapies for the prevention or even cure of ASCVD.
The March issue of Atherosclerosis contains several articles that investigated the prognostic value of biomarkers of inflammation or pathomechanisms related to inflammation.
Comparison of interleukin-6 and high-sensitivity C-reactive protein for cardiovascular risk assessment: Findings from the MESA study

Inflammation is a risk factor for major adverse cardiovascular events (MACE). Elevated levels of both high-sensitivity C-reactive protein (hsCRP) and interleukin-6 (IL6) have been associated with MACE. Using data from the Multi-Ethnic Study of Atherosclerosis (MESA) study cohort, Ferreira et al. compared IL6 to hsCRP for cardiovascular risk assessment.
IL6 and hsCRP were divided by their median values and 4 mutually exclusive groups based on median IL6 and hsCRP values (low-low, high-low, low-high and high-high) were created. Median follow-up was 14 years.
6614 (97%) participants had complete baseline IL6 and hsCRP data. The correlation between hsCRP and IL6 was modest. 3309 participants had high IL6 (≥1.2 pg/mL), and 3339 participants had high hsCRP (≥1.9 mg/L). Compared to participants with low IL6 and low hsCRP, those with high IL6 and high hsCRP were older, more frequently women, and with more cardiovascular co-morbidities. hsCRP outcome associations lost statistical significance when adjusting for IL6, while IL6 associations remained significant after adjusting for hsCRP. The C-index of Framingham score for did not improve with hsCRP but improved with IL6. Compared to participants with low IL6 and low hsCRP, those with high IL6, regardless of hsCRP, experienced an increased risk of MACE, heart failure and mortality.
In a diverse and asymptomatic population, IL6 showed a stronger association with atherosclerotic, heart failure and fatal outcomes than hsCRP.
Novel plasma biomarkers of coronary artery calcium incidence or progression: Insights from the prospective multi-ethnic Dallas Heart Study cohort
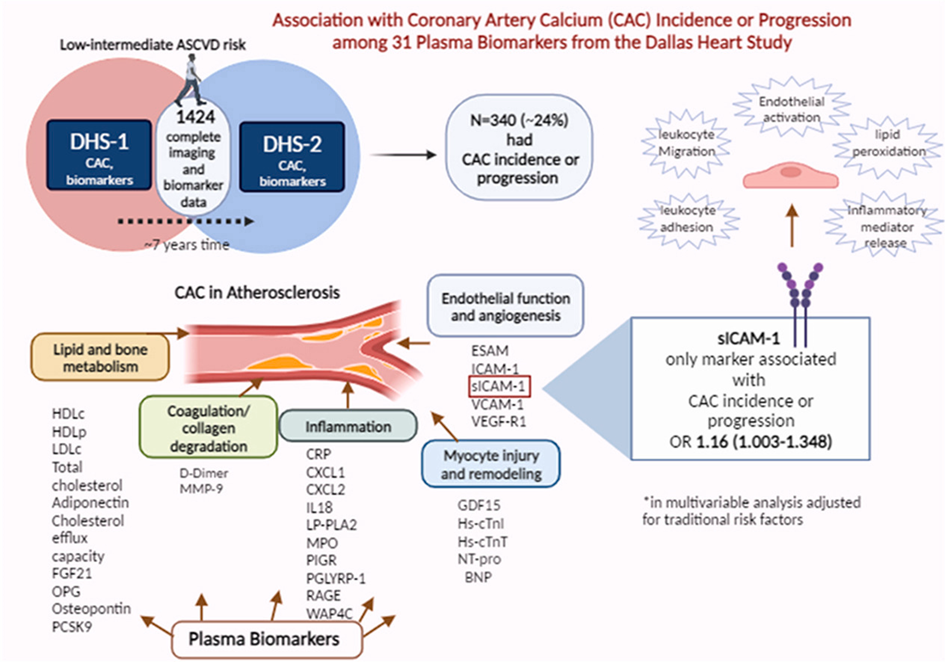
Coronary artery calcium (CAC), measured by non-contrast cardiac computed tomography (CCT), is strongly associated with atherosclerotic cardiovascular disease (ASCVD) risk in short and long-term follow-up, independent of traditional risk factors and regardless of age, gender, and ethnicity. Numerous plasma biomarkers have been linked to CAC incidence and progression including biomarkers of calcium-phosphate and lipid metabolism, inflammation, kidney function, and myocardial necrosis. In addition, the incorporation of novel biomarkers that are associated with CAC incidence and progression into existing risk calculators may improve the accuracy of ASCVD risk prediction, which is currently based only on traditional risk factors. Grinberg et al. aimed to examine a broad panel of plasma biomarkers among ambulatory individuals without prevalent ASCVD in the Dallas Heart Study (DHS) cohort, to explore their association with CAC incidence or progression to address the hypothesis that different pathophysiological mechanisms may underpin the development and/or progression of CAC in a diverse, primary prevention cohort. Participants of the DHS, who had their blood tested for 31 biomarkers reflecting multiple pathophysiological pathways, underwent 2 CCT assessments for CAC a median ∼7 years apart. The collected biomarkers were explored for association with CAC incidence or progression using univariate and multivariate analysis.
1424 participants were included. Over a 7-year interval between the two CAC measurements, 340 participants had CAC incidence or progression, 105 with incident CAC, and 309 with CAC progression. Although several plasma biomarkers were associated with CAC incidence or progression in a univariate model, only soluble intercellular adhesion molecule-1 (sICAM-1), related to atherosclerosis by the inflammatory pathway, remained independently associated in a multivariate model adjusted for traditional risk factors.
Further studies are needed to characterize the role of sICAM-1 in CAC evolvement to establish whether it has a pivotal mechanistic contribution or is rather an innocent bystander. Alternate measures of coronary atherosclerosis may be needed to elucidate contributors to atherosclerosis incidence or progression.
Human vascular smooth muscle cells utilise chymase for the atypical cleavage and activation of Interleukin-1β
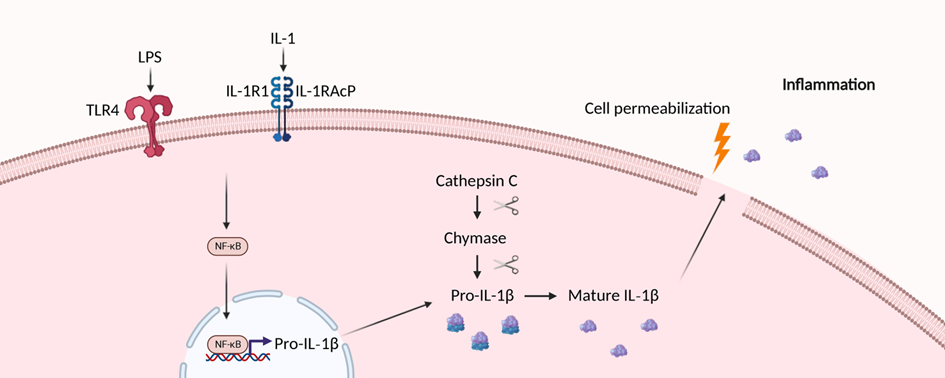
Atherosclerosis and other cardiovascular diseases (CVD) are both instigated and worsened by inflammation. The Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) proved that targeting the inflammatory cytokine Interleukin-1 β (IL-1β) only could reduce both cardiovascular events and death. However, due to the central role of IL-1β in host defense, blockade increased fatal infections, suggesting targeting key immune mediators over the long natural history of CVD is unsuitable.
To assess alternative mechanisms generating vascular inflammation that may identify more actionable targets, Morales-Maldonado et al. used primary human vascular smooth muscle cells (VSMCs) and a combination of biochemical, pharmacological and molecular biological techniques. Moreover, human carotid atherosclerotic plaques were assessed histologically.
VSMCs expressed and efficiently processed pro-IL-1β to the active form after receiving a single stimulus via Interleukin 1 receptor, type I (IL1R1) or Toll-like receptor 4 (TLR4). Importantly, pro-IL-1β processing did not utilise inflammasomes or caspases. Unusually, the authors found that cathepsin C-activated chymase was responsible for cleaving IL-1β in VSMCs, and provided evidence for chymase expression in cultured VSMCs and in the fibrous cap of human plaques. Chymase also efficiently cleaved and activated recombinant pro-IL-1β.
The results show that VSMCs are efficient activators of IL-1β that do not use canonical inflammasomes or caspases. Hence, targeting this alternative pathway for long-term treatment of CVDs, as it is not central to everyday host defense, could represent a novel strategy to safely control the inflammatory components driving atherogenesis and its complications.
Petri T. Kovanen provided an insightful editorial on this article.
Flagellar hook protein FlgE promotes macrophage activation and atherosclerosis by targeting ATP5B

In addition to conventional risk factors, increasing evidence has suggested that bacterial infections significantly contribute to the pathogenesis of atherosclerosis. Pseudomonas aeruginosa (P. aeruginosa) infections are strongly linked to the development of cardiovascular disease and atherosclerosis; however, the underlying mechanisms remain unclear. Li et al. previously confirmed that P. aeruginosa flagellar hook protein (FlgE), recognized as a key structure for flagellar synthesis and swimming motility, had immunostimulatory effects. FlgE administration triggers a proinflammatory response and immune reactions in the host. Structural analysis and experimental data showed that the interaction and bioactivity of FlgE primarily rely on peptides Pc-B (aa168-174) and Pc-F (aa303-309), which are disrupted by FlgEM mutations. In this study, the authors investigated the effects and mechanisms of action of FlgE on atherogenesis.
ApoE−/− mice were intravenously challenged with FlgE or FlgEM recombinant proteins for eight weeks. A murine model of chronic lung colonization was established using beads containing either mutable- or wild-type bacteria. Aortic sinus sections were stained to assess atherosclerosis progression. THP-1 macrophages exposed to FlgE or FlgEM were evaluated for their effects on lipid uptake and inflammation in vitro. Western blotting and pull-down assays were used to identify the binding proteins and signaling pathways involved, and specific blocking experiments were performed to confirm these effects.
The results showed that FlgE accelerated atherosclerosis progression by triggering lipid deposition and inflammatory responses in high-fat diet (HFD)-fed ApoE−/− mice. In comparison to infection with wild-type strain PAO1 (FlgE-proficient), infection with PAO1/flgEΔBmF (mutant PAO1 bacteria harboring mutations on both Pc-B and Pc-F sites, FlgE-deficient) resulted in reduced atherosclerosis. Mechanistic analysis indicated that FlgE exacerbated lipoprotein uptake and foam cell formation by upregulating Scavenger Receptor A1 (SR-A1) expression. Moreover, FlgE activated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Mitogen-activated protein kinases (MAPKs) signaling, which subsequently led to inflammatory responses in THP-1-derived macrophages. Pull-down assays revealed that FlgE directly interacted with ATP synthase F1 β subunit (ATP5B), whereas blocking ATP5B attenuated FlgE-induced responses in macrophages.
FlgE induces macrophage lipid uptake and pro-inflammatory responses mediated by ATP5B/NF-kB/AP-1 signaling, which eventually results in atherosclerosis. These findings support the development of therapeutic strategies for P. aeruginosa infection-induced atherosclerosis.
Metabolic reprogramming of immune cells by mitochondrial division inhibitor-1 to prevent post-vascular injury neointimal hyperplasia

Revascularization by angioplasty and stenting remain the main stay of treatment for many patients with coronary artery disease (CAD) and peripheral artery disease (PAD), but this procedure is associated with a significant risk of restenosis. The key contributor to post-angioplasty restenosis is tunica neointima hyperplasia and infiltration of inflammatory cells. As such, new treatments are needed to inhibit neointimal hyperplasia to prevent post-angioplasty restenosis in CAD and PAD patients to improve health outcomes in these patients. Crespo-Avilan et al. investigated whether modulating mitochondrial function using mitochondrial division inhibitor-1 (Mdivi-1) could reduce post-vascular injury neointimal hyperplasia by metabolic reprogramming of macrophages from pro-inflammatory to anti-inflammatory phenotype.
In vivo Mdivi-1 treatment of ApoE−/− mice fed a high-fat diet and undergoing carotid-wire injury decreased neointimal hyperplasia by 68%, reduced the number of plaque vascular smooth muscle cells and pro-inflammatory M1-like macrophages, and decreased plaque inflammation, endothelial activation, and apoptosis compared to control. Mdivi-1 treatment of human THP-1 macrophages shifted polarization from a pro-inflammatory M1-like to an anti-inflammatory M2-like phenotype, reduced monocyte chemotaxis and migration to CC-chemokine ligand 2 (CCL2) and macrophage colony stimulating factor (M-CSF) and decreased secretion of pro-inflammatory mediators. Treatment of pro-inflammatory M1-type-macrophages with Mdivi-1 metabolically reprogrammed them to an anti-inflammatory M2-like phenotype by inhibiting oxidative phosphorylation and attenuating the increase in succinate levels and correcting the decreased levels of arginine and citrulline.
Treatment with Mdivi-1 inhibits post-vascular injury neointimal hyperplasia by metabolic reprogramming macrophages towards an anti-inflammatory phenotype thereby highlighting the therapeutic potential of Mdivi-1 for preventing neointimal hyperplasia and restenosis following angioplasty and stenting in CAD and PAD patients.
Hypercholesterolemia exacerbates in-stent restenosis in rabbits: Studies of the mitigating effect of stent surface modification with a CD47-derived peptide
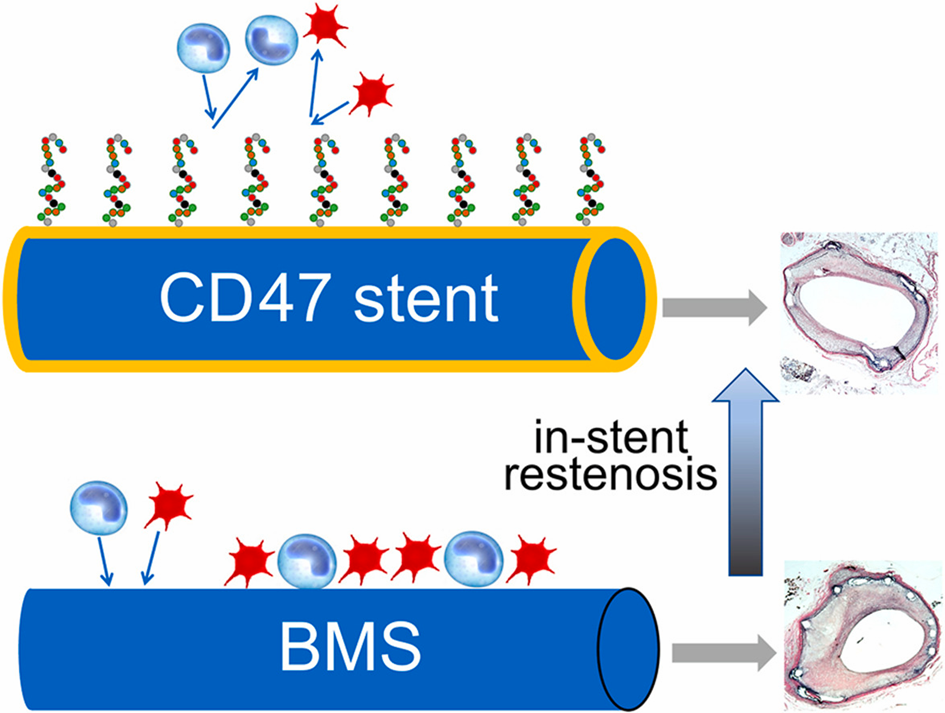
Hypercholesterolemia (HC) has previously been shown to augment the restenotic response in animal models and humans. However, the mechanistic aspects of in-stent restenosis (ISR) on a hypercholesterolemic background, including potential augmentation of systemic and local inflammation precipitated by HC, are not completely understood. Cluster of differentiation 47 (CD47) is a transmembrane protein known to abort crucial inflammatory pathways. Fishbein et al. examined the interrelation between HC, inflammation, and ISR and investigated the therapeutic potential of stents coated with a CD47-derived peptide (pepCD47) in the hypercholesterolemic rabbit model.
PepCD47 was immobilized on metal foils and stents using polybisphosphonate coordination chemistry and pyridyldithio/thiol conjugation. Cytokine expression in buffy coat-derived cells cultured over bare metal (BM) and pepCD47-derivatized foils demonstrated an M2/M1 macrophage shift with pepCD47 coating. HC and normocholesterolemic (NC) rabbit cohorts underwent bilateral implantation of BM and pepCD47 stents (HC) or BM stents only (NC) in the iliac location.
A 40 % inhibition of cell attachment to pepCD47-modified compared to BM surfaces was observed. HC increased neointimal growth at 4 weeks post BM stenting. These untoward outcomes were mitigated in hypercholesterolemic rabbits treated with pepCD47-derivatized stents. Compared to NC animals, inflammatory cytokine immunopositivity and macrophage infiltration of peri-strut areas increased in HC animals and were attenuated in HC rabbits treated with pepCD47 stents.
Augmented inflammatory responses underlie severe ISR morphology in hypercholesterolemic rabbits. Blockage of initial platelet and leukocyte attachment to stent struts through CD47 functionalization of stents mitigates the pro-restenotic effects of hypercholesterolemia.