Atherosclerosis newsletter
Highlighted articles from January issue Vol 388
Highlighted articles from February issue Vol 389
Newsletter by Editor in Chief Prof Arnold von Eckardstein, and Editorial Office Manager Simona Negrini
Join the Atherosclerosis social media community on Facebook (Atherosclerosis – Journal of the European Atherosclerosis Society) and Twitter (@ATHjournal) for scientific discussion and information about the journal.
Lipoprotein(a) (Lp(a)) is an important, mostly genetically determined risk factor for atherosclerotic cardiovascular diseases (ASCVD) as well as aortic valve calcification. Lp(a) experienced several waves of scientific and medical attention: a first one when it was discovered by Kare Berg as a genetically determined trait associated with coronary heart disease; a second one in the late 1980s and early 1990s when the apolipoprotein(a) gene was cloned and its variable number of repeat polymorphism was identified as the basis of the strong genetic determination, and cohort studies showed the independent association of elevated Lp(a) with increased risk of incident ASCVDs; a third one when genome wide association studies and Mendelian randomization studies established the LPA gene as a genetically causal risk factor of ASCVDs; and a forth one upon development of antisense oligonucleotides and siRNAs towards therapy to lower Lp(a) plasma levels. The current strong attention is also reflected by high numbers of submissions to Atherosclerosis addressing Lp(a). Some of the recently published papers are summarized below.
The association of lipoprotein (a) with coronary artery calcification: A systematic review and meta-analysis
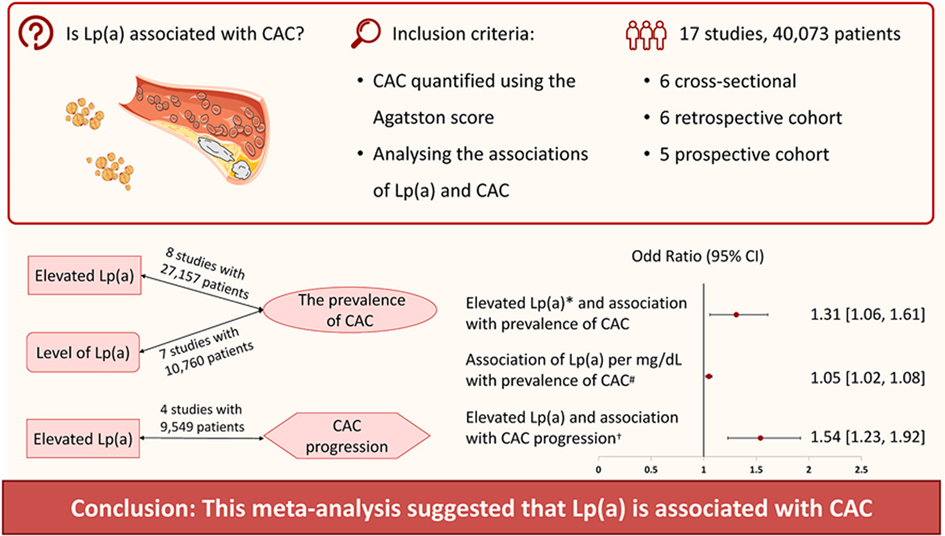
Coronary artery calcification (CAC) is a pathophysiological feature of atherosclerosis and closely associated with atherosclerotic plaque burden. Elevated lipoprotein(a) [Lp(a)] levels represent an independent risk factor for atherosclerosis. Despite previous studies, results on the association between Lp(a) and CAC are inconsistent. Qiu et al. aimed to assess the relationship between Lp(a) and CAC by exploring the association between elevated Lp(a) and CAC prevalence, Lp(a) levels and CAC prevalence, and the correlation between elevated Lp(a) and CAC progression.
PubMed, Web of Science, and EMBASE databases were searched, and studies exploring the association between serum Lp(a) and CAC (quantified using the Agatston score) were included in the analysis. Association between Lp(a) levels or elevated Lp(a) (higher than the cutoff values of 30 mg/dL, 50 mg/dL, or the highest quartile ranging from 33 to 38.64 mg/dL) and prevalence [CAC score >0 or >100, log (CAC score+1) >0] or progression (an increase in CAC score >0 or ≥100) of CAC were assessed.
40,073 individuals from 17 studies were included. The results show that elevated Lp(a) was associated with higher CAC prevalence and greater CAC progression. Furthermore, as a continuous variable, Lp(a) levels were positively correlated with the prevalence of CAC.
Further studies are required to explore whether Lp(a)-lowering therapy could prevent or inhibit CAC, ultimately reducing coronary artery disease risk.
Elevated lipoprotein(a) increases risk of subsequent major adverse cardiovascular events (MACE) and coronary revascularisation in incident ASCVD patients: A cohort study from the UK Biobank
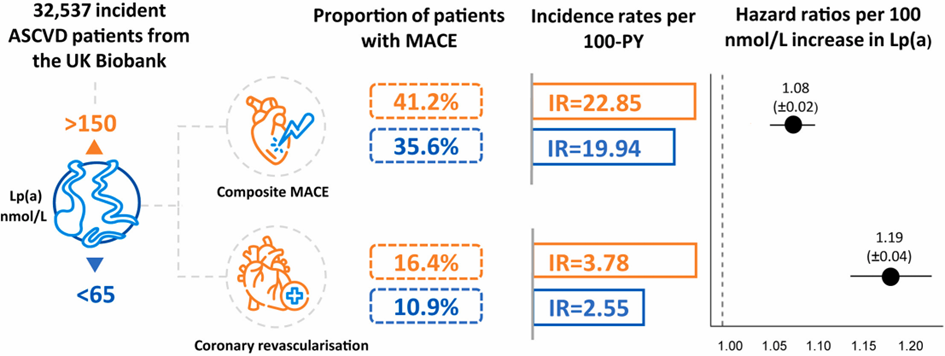
Elevated lipoprotein(a) [Lp(a)] is a genetic driver for atherosclerotic cardiovascular disease (ASCVD). With this observational cohort study of incident ASCVD patients, Welsh et al. aimed to provide insights into the associated risk of elevated versus normal Lp(a) levels on major adverse cardiovascular events (MACE) in an incident ASCVD cohort
MACE counts and incidence rates (IRs) per 100-person-years were reported for patients with normal and elevatedLp(a) within the first year after incident ASCVD diagnosis and overall follow-up. Cox proportional hazard models quantified the risk of MACE associated with a 100 nmol/L increase in Lp(a).
The study cohort included 32,537 incident ASCVD patients; 5204 with elevated and 22,257 with normal Lp(a). Of those with elevated Lp(a), 41.2% had a subsequent MACE, versus 35.61% with normal Lp(a). Within the first year of follow-up, the IRs of composite MACE and coronary revascularisation were significantly higher in patients with elevated versus normal Lp(a). This trend was also observed in the overall follow-up. Using time to first subsequent MACE, a 100 nmol/L increase in Lp(a) was associated with an 8.0% increased risk of composite MACE, and 18.6% increased risk of coronary revascularisation during the overall follow-up period.
The association of elevated Lp(a) with increased risk of subsequent MACE and coronary revascularisation highlights a population who may benefit from earlier and more targeted intervention for cardiovascular risk including Lp(a), particularly within the first year after ASCVD diagnosis. Proactive Lp(a) testing as part of routine clinical practice can help identify and better manage these higher-risk individuals.
Higher prevalence of coronary microvascular dysfunction in asymptomatic individuals with high levels of lipoprotein(a) with and without heterozygous familial hypercholesterolaemia
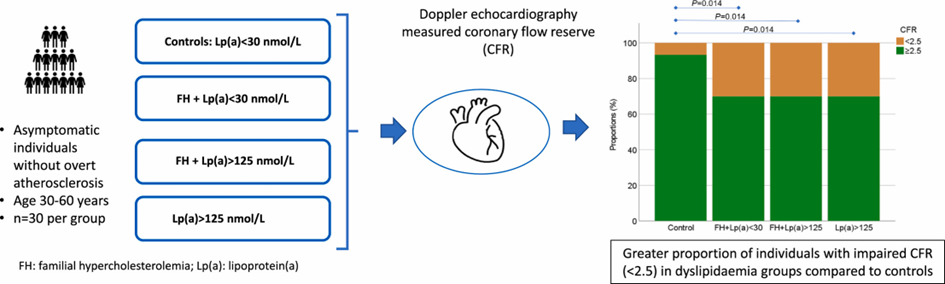
Patients with hereditary dyslipoproteinaemias, including high levels of lipoprotein(a) [Lp(a)], are at high cardiovascular risk. Microvascular dysfunction underlies many cardiovascular disease conditions, and little is known regarding its presence in individuals with high levels of lipoprotein(a) [Lp(a)]. Assessment of microvascular dysfunction in this context would be of clinical importance to predict future cardiovascular events and to guide primary preventive therapy. Wodaje et al. aimed to determine the frequency of microvascular dysfunction among these subjects with and without concomitant familial hypercholesterolemia (FH).
Four groups of asymptomatic individuals aged 30–59 years, without manifest cardiovascular disease, were recruited (n = 30 per group): controls with Lp(a) < 30 nmol/L, mutation-confirmed FH with Lp(a) < 30 nmol/L, or >125 nmol/L, and individuals with isolated Lp(a) > 125 nmol/L. Participants underwent evaluation of myocardial microvascular function by measuring coronary flow reserve (CFR) using transthoracic Doppler echocardiography, and of peripheral microvascular endothelial function using peripheral arterial tonometry.
The groups were balanced in age, sex, and body mass index. Each of the three dyslipoproteinaemic groups had a greater proportion of individuals with impaired coronary flow reserve, 30%, compared to 6.7% of controls. The median CFR levels did not differ significantly between the four groups. Cholesterol-lowering treatment time was longer in the individuals with normal than in those with impaired CFR in the FH + Lp(a) > 125 group, but not in the group with FH + Lp(a) < 30. There was no difference in peripheral endothelial function between the groups.
The results show that coronary microvascular dysfunction is more prevalent in asymptomatic individuals with isolated Lp(a) elevation and in heterozygous FH both with and without high Lp(a) compared to healthy controls. Cholesterol-lowering treatment could potentially prevent the development of microvascular dysfunction.