Atherosclerosis newsletter
Highlighted articles from April issue Vol 391
Newsletter by Editor in Chief Prof Arnold von Eckardstein, and Editorial Office Manager Simona Negrini
Join the Atherosclerosis social media community on Facebook (Atherosclerosis – Journal of the European Atherosclerosis Society) and Twitter (@ATHjournal) for scientific discussion and information about the journal.
The April issue of Atherosclerosis contains several articles reporting results of preclinical research on pathomechanisms and hence potential therapeutic targets of atherosclerosis, aortic aneurysm, or calcific aortic valve disease.
Low shear stress exacerbates atherosclerosis by inducing the generation of neutrophil extracellular traps via Piezo1-mediated mechanosensation
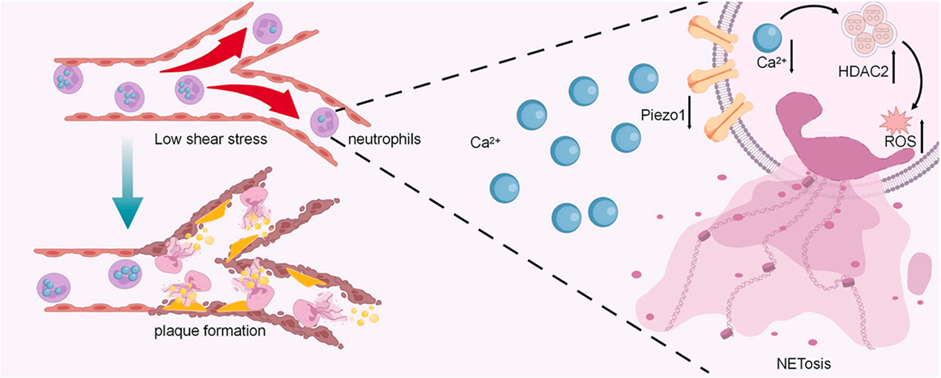
Atherosclerosis is a chronic lipid-driven inflammatory disease largely influenced by hemodynamics. Multiple factors have been found to participate in atherosclerosis, including endothelial dysfunction, oxidized low-density lipoproteins (oxLDLs), inflammation, and oxidative stress. Recent evidence supports neutrophils, the most abundant innate immune sentinels, as playing a role in atherosclerosis. One of the possible mechanisms is related to NETosis. In this process, activated neutrophils release neutrophil extracellular traps (NETs), which are fiber-like structures composed of decondensed chromatin and protein granules. Neutrophil extracellular trap (NET)-mediated inflammation plays an important role in atherosclerosis. Studies have shown that the morphology and activity of neutrophils are associated with blood flow shear stress (LSS). However, whether LSS can directly trigger NETosis is still unknown. In this study, Zhu et al. assessed the role of LSS in NET generation and atherosclerotic lesion development in vivo and investigated the underlying mechanism by which LSS promotes NET generation and injures endothelial cells in neutrophil-like HL-60 (dHL-60) cells and bone marrow-derived neutrophils (BMNs) in a parallel-plate flow chamber.
LSS was induced by partial ligation of the left carotid artery in high-fat diet-fed male ApoE−/− mice. To further validate the direct relationship between LSS and NET formation in vitro, differentiated human promyelocytic leukemia HL-60 cells and bone marrow-derived neutrophils were suspended in fluid flow under normal or low shear stress using a parallel-plate flow chamber system.
Four weeks after surgery, ligated carotid arteries had more lipid deposition, larger plaque area, and increased NET formation than unligated arteries. Inhibition of NETosis could significantly reduce plaque formation in ApoE−/− mice. In vitro, LSS could promote NET generation directly through downregulation of Piezo1, a mechanosensitive ion channel. Downregulation of Piezo1 could activate neutrophils and promote NETosis in static conditions. Conversely, Yoda1-evoked activation of Piezo1 attenuated LSS-induced NETosis. Mechanistically, downregulation of Piezo1 resulted in decreased Ca2+ influx and increased histone deacetylase 2 (HDAC2), which increased reactive oxygen species levels and led to NETosis. LSS-induced NET generation also promoted apoptosis and adherence of endothelial cells.
This study uncovers the essential role of Piezo1-mediated mechanical signaling in NET generation and plaque formation, which provides a promising therapeutic strategy for atherosclerosis.
Identification of circular RNA hsa_circ_0034621 as a novel biomarker for carotid atherosclerosis and the potential function as a regulator of NLRP3 inflammasome
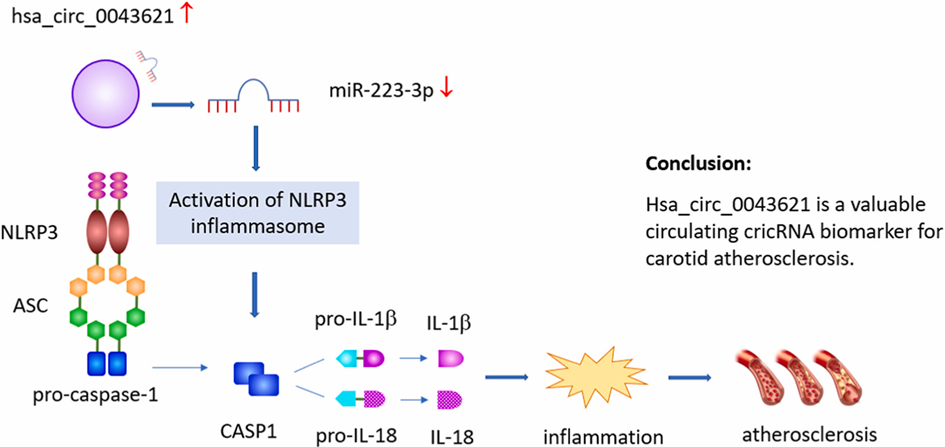
NLRP3 inflammasome plays a key role in vascular inflammation and atherosclerosis. Circular RNAs (circRNAs) are involved in disease development by regulating gene expression, and have emerged as promising novel disease biomarkers. Yan et al. aimed to identify the NLRP3 inflammasome-associated circRNA biomarkers of carotid atherosclerosis.
Based on the differential expression profiles of circRNAs in patients with carotid artery plaque (CAP) and healthy controls, hsa_circ_0043621, hsa_circ_0051995, and hsa_circ_0123388 were screened and validated using real-time quantitative polymerase chain reaction (RT-qPCR). Potential circRNA-miRNA-mRNA interactions were explored using a luciferase assay. The biological roles of the validated circRNAs were investigated in human umbilical vein endothelial cells (HUVECs) using Western blotting, transwell, and CCK-8 assays. Clinical significance was assessed using receiver operating characteristic (ROC) curves and logistic regression analysis.
The expression levels of all candidate circRNAs were significantly higher in patients with CAP than in controls, which was consistent with the results of the microarray analysis. Overexpression of hsa_circ_0043621 significantly increased the expression of NLRP3, induced migration of HUVECs, and inhibited cell proliferation. hsa_circ_0043621 demonstrated reasonable diagnostic accuracy for CAP detection and increased intima-media thickness (IMT). hsa_circ_0043621 upregulation was an independent predictor of an increased risk of CAP and increased IMT.
hsa_circ_0043621 is a valuable circulating biomarker of carotid atherosclerosis and may contribute to its pathogenesis by regulating the NLRP3 inflammasome.
Ganoderic acids alleviate atherosclerosis by inhibiting macrophage M1 polarization via TLR4/MyD88/NF-κB signaling pathway

Atherosclerosis (AS) is a chronic inflammatory disease characterized by lipid infiltration and plaque formation in blood vessel walls. Ganoderma lucidum (G. lucidum) is a traditional Chinese medicine with various pharmacological activities and low toxicity. Previous studies showed the potential therapeutic role of G. lucidum extracts on AS. Ganoderic acids (GA) are a class of the bioactive constituents of Ganoderma triterpenoid. GA were found to decrease the inflammatory response in renal ischemia reperfusion injury. Moreover, ganoderic acid A, one compound of GA, reduced the total cholesterol content in blood and liver of non-alcoholic fatty liver disease (NAFLD) rats. Based on these data, Quan et al. speculated that GA might be the active ingredients in G. lucidum to alleviate the development of AS and might be developed as candidate drug to prevent and treat AS.
ApoE–/- mice were fed a high-cholesterol diet and treated with GA for 16 weeks to induce AS. Network pharmacological analysis was performed to predict the anti-atherosclerotic mechanisms. An in vitro cell model was used to explore the effect of GA on macrophage polarization and the possible mechanism involved in bone marrow derived macrophages (BMDMs) and RAW264.7 cells stimulated with lipopolysaccharide or oxidized low-density lipoprotein.
It was found that GA at 5 and 25 mg/kg/d significantly inhibited the development of AS and increased plaque stability, as evidenced by decreased plaque in the aorta, reduced necrotic core size and increased collagen/lipid ratio in lesions. GA reduced the proportion of M1 macrophages in plaques, but had no effect on M2 macrophages. In vitro experiments showed that GA (1, 5, 25 μg/mL) significantly decreased the proportion of CD86+ macrophages and the mRNA levels of interleukin 6 and 1β (IL-6, IL-1β), and monocyte chemoattractant protein-1 (MCP-1) in macrophages. GA inhibited M1 macrophage polarization by regulating Toll-like receptor 4/myeloid differentiation primary response 88/nuclear factor κappa B (TLR4/MyD88/NF-κB) signaling pathway. This study demonstrated that GA play a role in plaque stability and macrophage polarization. GA exert the anti-atherosclerotic effect partly by regulating TLR4/MyD88/NF-κB signaling pathways to inhibit M1 polarization of macrophages.
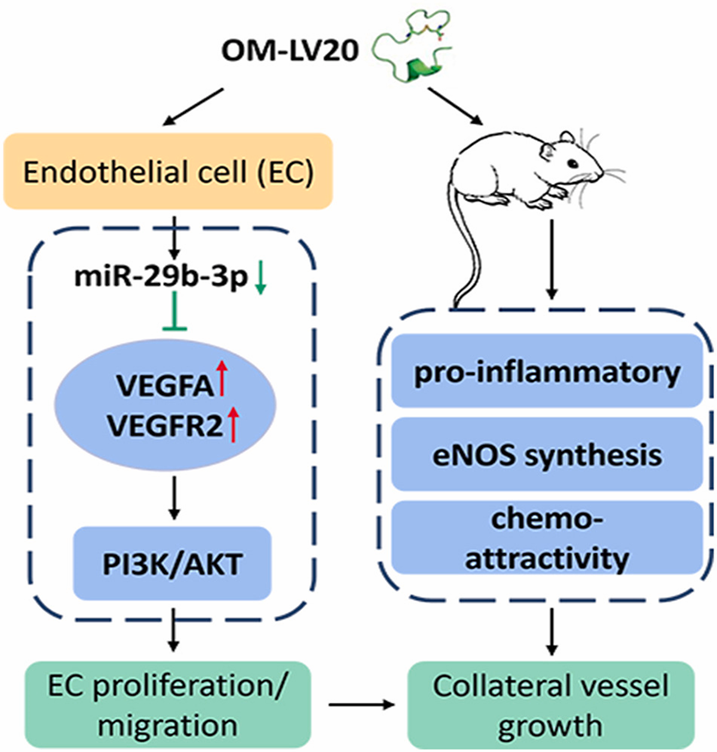
Peptide OM-LV20 promotes arteriogenesis induced by femoral artery ligature via the miR-29b-3p/VEGFA axis
Under ischemic conditions, arteriogenesis (also known as the collateral vessel growth), the enlargement of pre-existing interconnecting arterioles and conductance collateral arteries, can enhance perfusion of ischemic tissues. Therefore, therapeutic arteriogenesis, as a promising direction for the therapy of cardiovascular occlusive disease, is the induction of arteriogenesis to promote collateral vessel growth. However, pharmacological or biological approaches to stimulate functional collateral vessels remain elusive. Identifying new drug targets to promote and explore the underlying mechanisms for therapeutic arteriogenesis is necessary.
OM-LV20, a small-molecule peptide derived from the skin secretions of the frog Odorrana margaretae, wasshown to accelerate skin wound recovery. As vascular remodeling is implicated in the promotion of skin wound healing and alleviation of ischemia-reperfusion (I/R), Zhang et al. speculated that OM-LV20 had the potential to stimulate collateral vessel remodeling under ischemic conditions. The authors explored the function of OM-LV20 peptide application on collateral vessel growth using a rat model of hindlimb ischemia. They also investigated the potential mechanisms underlying the regulation of miRNAs and possible downstream signaling pathways by OM-LV20.
Peptide OM-LV20 (20 ng/kg) was administered for 7 consecutive days in a rat hindlimb ischemia model, collateral vessel growth was assessed by H&E staining, liquid latex perfusion, and specific immunofluorescence. In vitro, the effect of OM-LV20 on human umbilical vein endothelial cells (HUVEC) proliferation and migration was assessed. After transfection, quantitative real-time polymerase chain reaction, in situ-hybridization and dual luciferase reporter assay were performed to assess effective miRNAs and target genes. The proteins related to downstream signaling pathways were detected by Western blot.
OM-LV20 significantly increased visible collateral vessels and endothelial nitric oxide synthase (eNOS), together with enhanced inflammation cytokine and monocytes/macrophage infiltration in collateral vessels. Inhibition of microRNA miR-29b-3p enhanced proliferation and migration of HUVEC, as well as the expression of vascular endothelial growth factor A (VEGFA). OM-LV20 also promoted migration and proliferation of HUVEC, and VEGFA expression was mediated via inhibition of miR-29b-3p. Furthermore, OM-LV20 influenced the protein levels of VEGFR2 and phosphatidylinositol3-kinase (PI3K)/AKT and eNOS in vitro and in vivo.
OM-LV20 enhanced arteriogenesis via the miR-29b-3p/VEGFA/VEGFR2-PI3K/AKT/eNOS axis, highlighting the application potential of exogenous peptide molecular probes through miRNA, which could promote effective therapeutic arteriogenesis in ischemic conditions.