The availability of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, alirocumab and evolocumab, for use in the clinic has highlighted the need for practical guidance. In response, this Joint Task Force of the European Society of Cardiology/
European Atherosclerosis Society (ESC/EAS) has provided a document to aid clinicians in the appropriate use of these novel treatments.1 In balancing the clinical need for these treatments with their cost, the Task Force has not surprisingly taken a conservative approach, especially given the lack of clinical outcomes evidence to date.
Who to target?
Taking account of the licensed indications for these PCSK9 inhibitors, the Task Force has decided to focus on very high cardiovascular risk patients. The Task Force has prioritized three patient groups:
- Secondary prevention patients, including those with rapid progression of atherosclerotic cardiovascular disease (ASCVD, defined as repeated events within 5 years), or with diabetes with target organ damage or with a major risk factor
- Patients with familial hypercholesterolaemia (FH) without ASCVD ± additional risk factors (those with ASCVD are included above)
- Patients in either of the above groups with verified statin intolerance, with substantially elevated levels of plasma low-density lipoprotein cholesterol (LDL-C) despite maximally tolerated LDL-lowering therapy (efficacious statin such as rosuvastatin or atorvastatin with ezetimibe, or ezetimibe alone in the case of statin intolerant patients) (Table 1).
The Task Force recognizes that patients with severe chronic kidney disease (glomerular filtration rate <30 mL/min/1.73 m2) are also categorized as being at very high risk,2 but due to the lack of clinical trial data cannot make recommendations for the use of PCSK9 inhibitors in this group. Moreover, as shown by the SHARP trial, the contribution of ischaemic events to the ASCVD burden in this patient population is relatively minor compared with other patients groups.3 PCSK9 inhibitors are also indicated for use in patients with homozygous FH with substantially elevated LDL-C levels despite maximally tolerated therapy including lipoprotein apheresis, with the exception of patients with negative-negative LDLR mutations.
Table 1. Very high cardiovascular risk patients to consider for PCSK9 inhibitor treatment
| Patient group | LDL-C thresholds for consideration of PCSK9 inhibitor treatment |
| Very high risk, as defined by the Joint Task Force (2016)2 ASCVD, or patients with diabetes with target organ damage such as proteinuria, or patients with diabetes and a major risk factor such as marked hypercholesterolaemia or marked hypertension. | LDL-C >3.6 mmol/L (>140 mg/dL) For patients with rapid progression of ASCVD, LDL-C >2.6 mmol/L (>100 mg/dL) |
| Heterozygous FH Without ASCVD Without ASCVD, with additional risk factors With ASCVD | LDL-C >5.0 mmol/L (>200 mg/dL) LDL C >4.5 mmol/L (>175 mg/dL) LDL C >3.6 mmol/L (140 mg/dL) |
| Either of the above groups with verified statin intolerance | As above |
The Task Force emphasizes the need for specialized verification of statin intolerance – commonly referred to as statin associated muscle symptoms (SAMS), as in the EAS Consensus Panel statement.4 This recommendation is made against a background on ongoing controversy regarding the veracity of SAMS as a clinical entity, as discussed in a recent review.5 While the incidence of muscle symptoms is similar among statin-treated and placebo-treated patients across 26 long-term trials involving 170,000 patients, in observational trials and registries, 7-29% of patients have reported muscle symptoms. 4 It is acknowledged that these may not always be related to statin use, as shown by the GAUSS-3 trial.6 In this trial, patients who were unable to tolerate at least 3 statins (the majority of patients, 82%), 2 statins (one of which was atorvastatin ≤10 mg/day), or had a history of marked creatine kinase elevation with muscle symptoms while on a statin, were initially randomly allocated to treatment with atorvastatin (20 mg/day) or placebo for 12 weeks, and then crossed over to the alternative treatment. While 43% discontinued atorvastatin (but not placebo) due to intolerable muscle symptoms, 27% of patients reported similar symptoms with placebo but not atorvastatin. Despite this, in routine practice, SAMS is a real clinical issue, as supported by evidence from multinational studies.4,7 Thus, for those very high risk patients with SAMS, effective therapeutic options beyond ezetimibe may be considered.
What LDL-C thresholds?
There has been much debate regarding the appropriate LDL-C thresholds for consideration of PCSK9 inhibitor treatment. The focus has been on those patients who require more than 50% reduction in LDL C levels in order to attain the ESC/EAS recommended LDL-C goal (i.e. <1.8 mmol/L or <70 mg/dL in patients with ASCVD or <2.6 mmol/L or <100 mg/dL in patients without ASCVD). LDL-C thresholds summarised in Table 1 were based on the premise that the absolute reduction in plasma LDL-C is a critical determinant of the absolute reduction in risk of a cardiovascular event. The Task Force recognizes that to set a value for the absolute risk level is extremely difficult and somewhat arbitrary given the paucity of data on which to base decision making. Ultimately, the mission of this paper is to provide practical guidance for the use of PCSK9 inhibitors in the clinic. To help with treatment decisions, the Task Force has provided clinical algorithms; the algorithm given below provides an overview on the management of all three patient groups; readers are also referred to the publication, available free to download at: https://www.ncbi.nlm.nih.gov/pubmed/27789571
Going forward
As emphasized by the Task Force, this practical guidance is a conservative approach to the management of elevated LDL-C levels in very high risk patients, bearing in mind the lack of outcomes evidence with these novel treatments.
Recent publication of the GLAGOV (Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound) study using intravascular ultrasound provides some insights. In this study in patients with symptomatic coronary artery disease (20-50% stenosis in a target vessel) and baseline LDL-C 2.4 mmol/L (92 mg/dl) on stable, intensive to moderate statin therapy. The addition of the PCSK9 inhibitor evolocumab 420 mg monthly resulted in greater LDL-C lowering than statin alone (0.95 mmol/L [36.6 mg/dl] vs. 2.4 mmol/L [93 mg/dl]), far below the current ESC/EAS recommended goal. Furthermore, a greater proportion of patients regressed on evolocumab than statin alone (64% vs. 47%).8 Whether this translates to clinical outcomes benefit, however, is not yet known.
2017 will present us with the first evidence to fill this gap, with the eagerly anticipated results of the outcomes study FOURIER with evolocumab.9 However, we have to bear in mind that the patients included in FOURIER (and in ODYSSEY OUTCOMES with alirocumab, still ongoing10, may differ somewhat from those groups recommended by the Task Force, both with respect to baseline LDL-C levels and background LDL-C lowering therapy. In this (and most) trials with the PCSK9 inhibitors, treatment was given against a statin background, rather than the combination of statin plus ezetimibe, which is the setting recommended by this Task Force. The availability of data from these highly relevant outcomes studies, will enable the Task Force to make a more accurate appraisal of the evidence so as to update this highly relevant practical document.
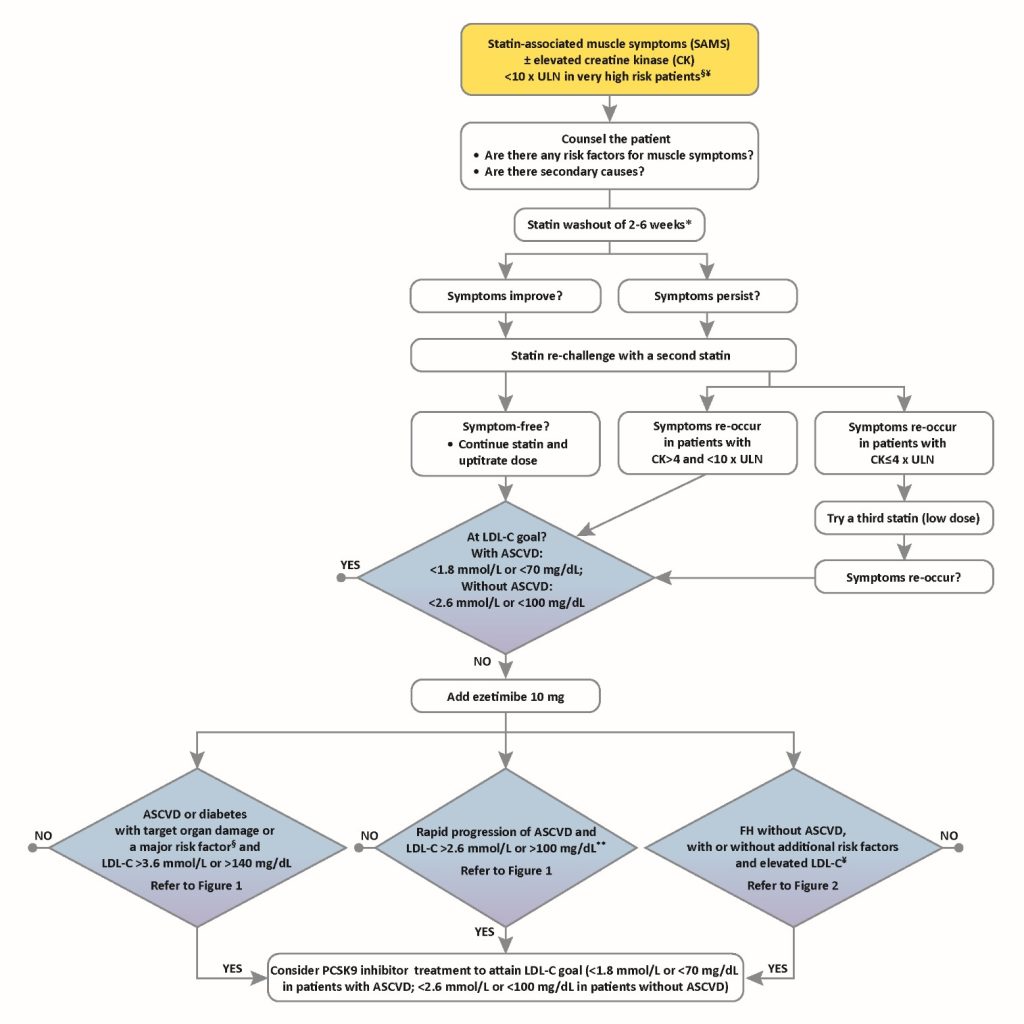
Notes to this algorithm:
§ Very high risk as defined by the Sixth Joint Task Force (2016)2 as documented clinical ASCVD includes previous acute myocardial infarction, acute coronary syndrome, coronary revascularization and other arterial revascularization procedures, stroke and transient ischaemic attack, aortic aneurysm and peripheral arterial disease. Unequivocally documented ASCVD on imaging includes plaque on coronary angiography or carotid ultrasound. Diabetes with evidence of target organ damage such as proteinuria, or with a major risk factor such as marked hypercholesterolaemia or marked hypertension.
¥ Additional risk factors that indicate a very high cardiovascular risk include diabetes mellitus, elevated lipoprotein(a) >50 mg/dL, marked hypertension and premature familial ASCVD (<55 years in males and <60 years in females)
*Suggested statin washout is dependent of creatine kinase (CK) elevation, i.e. 2-4 weeks if CK elevation is <4 x upper limit of normal (ULN), and 6 weeks if CK elevation is > 4 x ULN.
** Rapid progression of ASCVD is defined as repeated acute coronary syndromes, repeated unplanned coronary revascularizations, or repeated ischaemic strokes within 5 years of the index event
References
1. Landmesser U, Chapman MJ, Farnier M et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J 2016. pii: ehw480. [Epub ahead of print]
2. Catapano AL, Graham I, De Backer G et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2016 Aug 27. pii: ehw272. [Epub ahead of print].
3. Baigent C, Landray MJ, Reith C et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181-92.
4. Stroes ES, Thompson PD, Corsini A et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012-22.
5. Collins R, Reith C, Emberson J et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532-61.
6. Nissen SE, Stroes E, Dent-Acosta RE et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 2016;315:1580-90.
7. Hovingh GK, Gandra SR, McKendrick J et al. Identification and management of patients with statin-associated symptoms in clinical practice: a clinician survey. Atherosclerosis. 2016;245:111-7.
8. Nicholls SJ, Puri R, Anderson T et al. Effect of evolocumab on progression of coronary disease in statin-treated patients. The GLAGOV randomized clinical trial. JAMA. doi:10.1001/jama.2016.16951. Published online November 15, 2016.
9. Sabatine MS, Giugliano RP, Keech A et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J 2016;173:94-101.
10. Schwartz GG, Bessac L, Berdan LG et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J 2014;168:682–9.