Quantifying atherogenic lipoproteins: what clinicians and laboratory personnel need to know. New pragmatic guidance from this Joint Consensus Initiative from the European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM).
Quantifying atherogenic lipoproteins – pragmatic guidance from the EAS/EFLM Joint Consensus Initiative
Low-density lipoprotein cholesterol (LDL-C) has long been considered the primary target for lipid-modifying strategies to lower risk for atherosclerotic cardiovascular disease (ASCVD). Indeed, the 2019 Joint European Society of Cardiology/European Atherosclerosis Society (ECS/EAS) Guidelines for Dyslipidaemia Management reinforce this, stating that LDL-C levels should be lowered as much as possible to prevent ASCVD, especially in high and very high-risk patients.1 There are now highly efficacious therapies available for lowering LDL-C to very low levels and more in development. Yet it is also evident that even when LDL-C levels are below current guideline-recommended goals, high and very high-risk patients continue to experience cardiovascular events.2,3 This residual cardiovascular risk may be at least partly addressed by targeting other atherogenic lipoproteins; the critical question is which?
Yet another issue to consider is laboratory diagnostics of atherogenic lipoproteins, including LDL-C. There has been much uncertainty regarding which atherogenic lipoproteins should be measured, both for initial assessment and follow-up of treatment efficacy, and under what conditions (fasting versus nonfasting). Additionally, in the era of attainable very low LDL-C levels, reliability and standardization of assessment become increasingly relevant.4
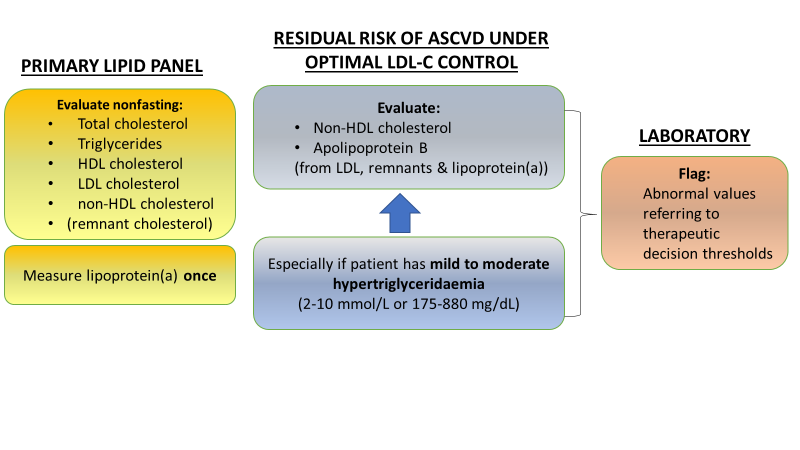
In 2016 and 2018, a Joint Consensus Initiative from the EAS and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) published two statements which addressed challenges in laboratory diagnostics of atherogenic lipoproteins.5,6 This Joint Consensus Initiative has now published pragmatic guidance for clinicians based on these statements.7 This new report addresses the key points of the two statements, so as to resolve residual uncertainty and improve the harmonization of lipoprotein measurement across the clinic and laboratory. This guidance takes key questions (Table 1) and provides practical advice and recommendations (as summarized in Figure 1).
| Table 1. EAS/EFLM Joint Consensus Initiative: Questions and guidance | |
| Question | EAS/EFLM Guidance |
| 1. What is the primary lipid panel for estimating cardiovascular risk? | • The Panel recommends total cholesterol, triglycerides, HDL-C, LDL-C, and calculated non-HDL-C (total – HDL-C), with the option of also reporting – if directly measured LDL-C is used – calculated remnant cholesterol. • Where cost is a major issue, a minimal lipid profile of total cholesterol and triglycerides can be considered. • Lipoprotein(a) [Lp(a)] should be measured once in all individuals. • Apolipoprotein (apo) B may be included in selected cases. |
| 2. LDL-C: calculated or direct measurement? | • For calculated LDL-C, the Martin-Hopkins modified equation may be preferable over the standard Friedewald equation, especially in individuals with low LDL-C (<1.8 mmol/L or <70 mg/dL) and/or triglycerides of 2.0–4.5 mmol/L (175-400 mg/dL), and in nonfasting samples. • Direct LDL-C assays should be used to calculate remnant cholesterol, and if triglycerides exceed ≥4.5 mmol/L (400 mg/dL), or ≥4.0 mmol/L (350 mg/dL) in some countries. • Lp(a)-cholesterol correction of LDL-C should be applied in patients i) with known or suspected high Lp(a), or ii) who show a poor response to LDL-lowering therapy. |
| 3. What other atherogenic lipoproteins can be measured? | • ApoB is recommended for risk assessment and may be preferred over non HDL-C, especially in patients with any of the following:triglycerides 2-10 mmol/L (175-880 mg/dL), diabetes, obesity or metabolic syndrome, or LDL-C <1.8 mmol/L (<70 mg/dL). • ApoB can be measured in nonfasting samples and is not affected by variability in triglycerides |
| 4. Fasting or nonfasting blood samples? | • Fasting is not routinely required for the determination of a lipid profile. • If a nonfasting lipid profile shows triglycerides ≥4.5 mmol/L (400 mg/dL), a repeat fasting lipid profile could be performed; however, this is not a requirement. |
| 5. Follow-up of LDL-targeted lipid-lowering therapy: what measures? | • Ideally, follow-up to assess treatment efficacy should use the same LDL-C method (and preferably the same laboratory). • If the LDL-C value is close to therapeutic decision thresholds, measurement should be repeated (at least twice) and results averaged. • Either non-HDL-C or apoB should be considered as an index of the efficacy of treatment targeting LDL-C. Non-HDL-C has practical advantages. • Non-HDL-C may be considered rather than direct LDL-C measurement if triglycerides are ≥4.5 mmol/L (400 mg/dL). |
| 6. How should lipid values be reported? | • Flagging of lipid profiles on laboratory reports should always be based on decision thresholds; reference intervals are relevant for children. • Extremely high levels should automatically trigger alerts to investigate |
Figure 1. Quantifying atherogenic lipoproteins for lipid lowering strategies, based on the EAS/EFLM Consensus Initiative recommendations
What and how to measure?
While guidelines and consensus1,5,6 reaffirm that LDL-C remains the primary target of lipid-lowering therapy, a more comprehensive approach is needed (Table 1). The primary lipid panel for assessment of global ASCVD risk should therefore include a total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL C), LDL-C and non-HDL-C (calculated as total – HDL-C), ideally also with remnant cholesterol if direct LDL-C measurement is used (calculated as total cholesterol – HDL-C – LDL C). Assessment of LDL-C alone is inadequate to assess the total cholesterol load; moreover, new evidence shows that one-third of total cholesterol in the plasma is carried in remnant lipoproteins.8
Another important lipoprotein to consider is lipoprotein(a) [Lp(a)], given evidence that establishes a causal role in ASCVD.9,10 In accordance with the 2019 ESC/EAS dyslipidaemia guidelines1, Lp(a) should be measured once in all individuals, especially in patients with premature ASCVD (men <55 years, women <60 years), family history of premature ASCVD and/or elevated Lp(a), familial hypercholesterolaemia, or recurrent ASCVD despite optimal lipid-lowering treatment.
There are, however, practical issues to consider, especially in lower income countries where affordability is an important driver of control of dyslipidaemia. In these regions, measurement of LDL-C by direct methods is often relatively costly and resource-intensive. Therefore, a ‘minimal lipid profile’ including total cholesterol and triglycerides is recommended by the Joint Consensus Initiative.
Both non-HDL-C or apolipoprotein (apo) B are recommended as secondary lipid targets when LDL-C goal is achieved, particularly for patients with mild to moderate hypertriglyceridaemia (2-10 mmol/L or 175-880 mg/dL), diabetes, obesity or metabolic syndrome.1 Non-HDL-C may be more accurate than either calculated (Friedewald equation) or direct measurement of LDL-C, especially if triglycerides exceed ≥4.5 mmol/L (400 mg/dL), and offers practical advantages in the clinic. ApoB – which is a direct measure of the total number of lipoproteins causing cardiovascular disease – has shown superior efficacy to both LDL-C and non-HDL-C.5 As biological variability is low, repeated measurement of apoB may be more reliable than calculated non-HDL-C. Moreover, with the increased availability of automated immunoassays and improved international standardization,11 there is a case for recommending the use of apoB. As one expert group recently concluded: ’….apoB unifies, amplifies, and simplifies the information from conventional lipid markers as to the atherogenic risk attributable to the apoB lipoproteins.’12
To date, either non-HDLC or apoB should be considered as an index of the efficacy of treatment targeting LDL-C. Lipid profiles should routinely report non-HDL-C.
When to measure?
The need for fasting lipid assessment has been a long-standing controversy, especially with realization that the nonfasting state predominates during a 24-hour period in most individuals. The 2016 Joint EAS/EFLM statement addressed this issue and concluded that there was no scientific evidence to support the use a fasting rather than nonfasting sample in lipid testing for ASCVD risk prediction.6 Furthermore, the use of nonfasting samples confers several advantages for patients, especially in those with diabetes where it would minimize the risk of hypoglycaemia (as opposed to fasting samples). Therefore, the current EAS/EFLM Joint Consensus Initiative reaffirms the use of nonfasting samples in lipid testing. The only possible exception to this recommendation is when triglycerides exceed 4.5 mmol/L (400 mg/dL), when a repeat fasting lipid profile is suggested; however, even in this situation a fasting lipid profile is not required.
How to report?
Another consideration is how to report lipid testing results to aid clinicians in their routine practice. Flagging of abnormal lipids should be based on therapeutic decision threshold. In addition, this EAS/EFLM panel recommends that extremely abnormal life-threatening test results should be flagged with special alert to clinicians to quickly investigate these patients as a priority.
Conclusion
This new report from the EAS/EFLM Joint Consensus Initiative offers pragmatic guidance for the assessment of atherogenic lipoproteins for ASCVD risk assessment and treatment. Application by both clinicians and laboratory personnel will help to improve personalized management of patients at high risk of ASCVD.
References
1. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2019; doi: 10.1093/eurheartj/ehz455. [Epub ahead of print]
2. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713-22
3. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097-107.
4. da Silva PM, Duarte JS, von Hafe P, et al. Standardization of laboratory and lipid profile evaluation: A call for action with a special focus in 2016 ESC/EAS dyslipidaemia guidelines – Full report. Atheroscler Suppl 2018;31:e1-e12.
5. Langlois MR, Chapman MJ, Cobbaert C, et al; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and very low concentrations of LDL cholesterol. A Consensus Statement from EAS and EFLM. Clin Chem 2018;64:1006-33.
6. Nordestgaard BG, Langsted A, Mora S, et al; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) joint consensus initiative. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 2016;37:1944-58.
7. Nordestgaard BG, Langlois MR, Langsted A, et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from the EAS and EFLM. Atherosclerosis 2019; https://doi.org/10.1016/j.atherosclerosis.2019.12.005
8. Balling M, Langsted A, Afzal S, et al. A third of nonfasting plasma cholesterol is in remnant lipoproteins: Lipoprotein subclass profiling in 9293 individuals. Atherosclerosis 2019;286:97-104.
9. Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI Working Group Recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol 2018;71:177-192.
10. Kamstrup PR. Lipoprotein(a): the common, likely causal, yet elusive risk factor for cardiovascular disease. J Lipid Res 2017;58:1731-2.
11. Contois JH, Delatour V. Apolipoprotein B measurement: Need for standardization. J Clin Lipidol 2018;12:264-5.
12. Sniderman AD, Thanassoulis G, Glavinovic T et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol 2019;doi: 10.1001/jamacardio.2019.3780. [Epub ahead of print]