The challenge of peripheral arterial disease: how do we improve outcome?
Peripheral arterial disease (PAD) is a largely unrecognized complication of atherosclerotic cardiovascular disease (ASCVD). Yet PAD is very much an issue for clinicians in the 21st century, given that it typically presents in later life, usually in association with atherosclerotic complications in other vascular beds. Estimated to affect 10-20% of individuals aged 60 years or more,1,2 the prevalence of PAD will undoubtedly escalate in response to changing population demographics and lifestyle. Already in 2010, more than 202 million individuals were affected by PAD, reflecting almost a 25% increase since 2000, with two-thirds living in low- and middle-income countries.2 Whereas PAD increased by 13% in high-income regions over this decade, the rise in low to middle income regions was more than 2-fold (by 29%). Given that low to middle income regions have also seen larger increases in smoking and diabetes prevalence, both of which are powerful predictors of PAD,3 there is clearly an urgent need for action to avoid this burgeoning global pandemic.
As either an initial or subsequent manifestation of ASCVD, PAD (diagnosed as an ankle-brachial index ≤0.90) is associated with more than doubling of the 10-year rates for coronary events, and cardiovascular and all-cause mortality.4 Event rates are higher in individuals with ASCVD in multiple vascular beds (i.e. polyvascular disease).5 There is also evidence to suggest greater coronary plaque vulnerability in both culprit and nonculprit lesions in patients with PAD with or without other cardiovascular complications.6 Thus, the burden of PAD is substantial, both in terms of the impact on patient quality of life, as well as economic outcomes, the latter largely driven by the need for hospitalization and revascularization procedures.7-9
The main goals of treatment of patients with PAD are to reduce cardiovascular risk and improve functional capacity. Given the lack of awareness of PAD, however, undertreatment is an issue. The latest European guidelines recommend statin treatment in all patients with PAD, with low-density lipoprotein cholesterol (LDL-C) goals consistent with very high risk, i.e. < 1.8 mmol/L (<70 mg/dL) or a decrease by ≥50% if the initial LDL-C level is between 1.8 and 3.5mmol/L (70 and 135 mg/dL). Antiplatelet agents, angiotensin converting enzyme inhibitors/ angiotensin receptor blockers and antithrombotic agents are recommended for secondary prevention.3 In practice, however, management is less than optimal. For example, in the US National Health and Nutrition Examination Survey (1999-2004) based on an estimated 7.1 million people with PAD, only 30% received a statin, 25% an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, and only 36% aspirin.10 Even with evidence that attainment of guideline-recommended LDL-C goal is associated with improved outcome,11 adherence with statin therapy is often problematic.12
Thus, PAD represent a ‘perfect storm’ scenario when taking account of changing population demographics, and issues with diagnosis and treatment. Despite this, cardiovascular outcome studies have concentrated mainly on MI and stroke. Thus, new insights from the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk), which included a subgroup of patients with PAD, merit closer examination.
FOURIER: Evidence in PAD patients
The FOURIER trial showed that lowering LDL-C levels beyond guideline-recommended goals with the addition of the PCSK9 inhibitor evolocumab to intensive statin therapy reduces cardiovascular events in patients with clinical ASCVD.13 In a separate analysis, there was no evidence to suggest a lower threshold for clinical benefit.14 While the majority of patients in FOURIER had CHD, the trial also included a proportion of patients with PAD. Consequently, a key focus was to investigate whether the benefit observed in the overall study population would also translate to improved benefit in this PAD subgroup.
This analysis included 3,642 patients with symptomatic lower extremity PAD (defined as the presence of lower extremity intermittent claudication and an ankle brachial index <0.85, or prior peripheral revascularization or amputation for ischaemia), of whom 1,505 had no prior stroke or myocardial infarction (MI). The profile of these patients was consistent with that previously reported for PAD,2 with a higher prevalence of major risk factors compared with patients without PAD (for example, hypertension: 85% versus 79% in those without PAD; smoking: 36% versus 27%; diabetes mellitus: 43% versus 35%).15 The very high cardiovascular risk associated with PAD is reflected by absolute major cardiovascular event rates (MI, stroke or cardiovascular death; MACE] that were significantly higher than in individuals without PAD (16.8% versus 12.1% in the placebo groups, Kaplan-Meier rate at 2.5-years).
Treatment with evolocumab on top of statin resulted in comparable LDL-C reduction versus placebo to that observed in the total study population (decrease in LDL-C by 59% or 57 mg/dl [1.47 mmol/L] at 48 weeks versus statin alone), and led to a 27% (p=0.004) relative reduction in MACE event rates. The reduction in MACE events was even higher in PAD patients without prior MI or stroke (relative reduction 43%, p=0.0095, Table 1). As shown in the FOURIER study as a whole, the lower the level of achieved LDL-C the greater the benefit, with no lower threshold for benefit.
Evolocumab treatment was also associated with a 37% relative reduction in risk for major adverse limb events (MALE), defined as acute limb ischaemia, major amputation, or urgent peripheral revascularization for ischaemia; this reduction was even higher in PAD patients without a prior MI or stroke (relative reduction 57%, Table 1). Moreover, in patients with PAD alone without MI or stroke, treatment with evolocumab halved the combined endpoint of MACE or MALE (hazard ratio 0.52, 95% CI 0.35-0.76, p=0.0006); the absolute risk reduction was 6.3% over the 2.5-year Kaplan-Meier period (number needed to treat 16).
Over the limited trial duration, there was no evidence to suggest any safety issues with PCSK9 inhibition on top of statin therapy in PAD patients.
Table 1. FOURIER PAD subgroup analysis: Key outcomes
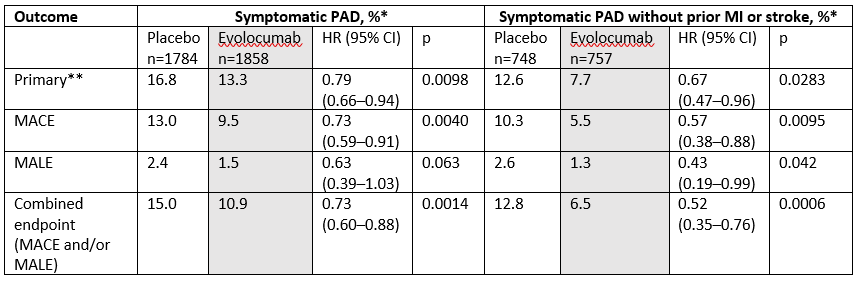
* 2.5 year Kaplan-Meier absolute event rates; ** Primary endpoint in FOURIER, defined as the composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization; MACE major adverse cardiovascular events (i.e. cardiovascular death, MI, or stroke); MALE major adverse limb events (i.e. acute limb ischemia, major amputation above the knee or below the knee, excluding forefoot or toe, or urgent revascularization (thrombolysis or urgent vascular intervention for ischemia))
Implications for clinical practice
Despite the limitations of subgroup analyses, there are highly relevant implications from this FOURIER analysis for clinical practice. First, the analysis reaffirms the high absolute risk of cardiovascular events in patients with symptomatic PAD. Second, the study shows that further lowering of LDL-C levels with the addition of a PCSK9 inhibitor provides further reduction in MACE in these patients. Third, these patients also derived benefit in terms of reduction in MALE commensurate with attainment of LDL-C levels beyond current goals. Not only is this finding unique, but it also lends support to a role for LDL-C in the aetiology of lower extremity ischaemic events. Finally, it is important to emphasize that, unlike antithrombotic treatment, the use of PCSK9 inhibition in PAD patients does not suggest potential safety concerns.3
In healthcare systems with increasingly finite budgets, the cost of treatment undoubtedly impacts clinical decision making. In updated practical guidance for the use of PCSK9 inhibition, a Joint Task Force from the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) recognized the very high cardiovascular risk of PAD, and its association with polyvascular disease.16 Both conditions were considered to provide additional indices of risk in patients with clinical ASCVD with substantial residual LDL-C burden despite treatment with statin plus ezetimibe (or ezetimibe alone in patients with statin intolerance). Evidence from this FOURIER analysis that the addition of a PCSK9 inhibitor also results in consistent benefits for limb ischaemia, amputation and peripheral revascularization, all of which incur substantial disability, morbidity and cost, warrant consideration in revisions of guidelines for prevention of ASCVD (Table 2).
| Table 2. Key implications |
| • PAD is a common but under-recognized and undertreated complication of ASCVD. • The prevalence of PAD is increasing as a result of changing population demographics and lifestyle. • The presence of PAD more than doubles cardiovascular morbidity and mortality. • Patients with PAD typically have other affected vascular territories and are at very high risk of cardiovascular events. • FOURIER has shown that further lowering of LDL-C levels below current guideline-recommended goals provides further clinical benefit in patients with symptomatic PAD, not only for reduction in cardiovascular events, but also, uniquely, reduction in lower extremity ischaemic events, including amputations. • These findings may warrant consideration in revisions of guidelines for prevention of ASCVD. |
References
- Sigvant B, Wiberg-Hedman K, Bergqvist D et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg 2007;45:1185-91.
- Fowkes FG, Rudan D, Rudan I et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329-40.
- Aboyans V, Ricco J-B, Bartelink M-LEL et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 2017; DOI: 10.1093/eurheartj/ehx095.
- Fowkes FG, Murray GD, Butcher I et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208.
- Subherwal S, Patel MR, Kober L et al. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol 2015;22:317-25.
- Bryniarski KL, Yamamoto E, Takumi H et al. Differences in coronary plaque characteristics between patients with and those without peripheral arterial disease. Coron Artery Dis 2017;28:658-63.
- Marrett E, DiBonaventura Md1, Zhang Q et al. Burden of peripheral arterial disease in Europe and the United States: a patient survey. Health Qual Life Outcomes 2013;11:175.
- Scully RE, Arnaoutakis DJ, DeBord Smith A et al. Estimated annual health care expenditures in individuals with peripheral arterial disease. J Vasc Surg 2017; doi: 10.1016/j.jvs.2017.06.102
- Smolderen KG, Wang K, de Pouvourville G et al. Two-year vascular hospitalisation rates and associated costs in patients at risk of atherothrombosis in France and Germany: highest burden for peripheral arterial disease. Eur J Vasc Endovasc Surg 2012;43:198-207.
- Pande RL, Perlstein TS, Beckman JA, Creager MA. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011;124:17-23.
- Lee JH, Ko YG, Shin DH et al. Attainment of low-density lipoprotein cholesterol goal after endovascular treatment is associated with reduced cardiovascular events in patients with peripheral arterial disease. J Vasc Surg 2016;63:756-63.
- Armstrong EJ, Chen DC, Westin GG et al. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J Am Heart Assoc 2014;3:e000697.
- Sabatine MS, Giugliano RP, Keech AC et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713-22.
- Giugliano RP, Pedersen TR, Park JG et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 2017;390:1962-71.
- Bonaca MP, Nault P, Giugliano RP et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation 2017 Nov 13. doi: 10.1161/CIRCULATIONAHA.117.032235. [Epub ahead of print]
- Landmesser U, Chapman MJ, Stock JK et al. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J 2017 Oct 16. doi: 10.1093/eurheartj/ehx549. [Epub ahead of print]