At the end of the ODYSSEY…what now for PCSK9 inhibition in practice?
One year ago, the first of the cardiovascular outcomes studies with a PCSK9 inhibitor, FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk), was published.1 This was widely regarded as a landmark trial, showing significant reduction in cardiovascular outcomes associated with incremental lowering of low-density lipoprotein cholesterol (LDL-C) with evolocumab against a background of intensive statin therapy in patients with stable atherosclerotic cardiovascular disease (ASCVD). Subsequent analyses have shown that the clinical benefits of PCSK9 inhibition were more pronounced in patients with additional indices of risk severity, such as peripheral artery disease, recurrent myocardial infarction (MI) and multivessel disease.2,3
The duration of FOURIER, however, was short (median 26 months). This is relevant as it is recognized that there is a lag in treatment benefit associated with LDL-C lowering therapy, including statins, being less in the first year of treatment, and thereafter increasing to a similar magnitude of benefit over the following years on therapy.4 Thus, the decision to expand patient recruitment and shorten the study follow-up is a likely contributor to the lack of significant benefit on cardiovascular mortality observed in FOURIER.
This March the second of the PCSK9 inhibitor outcomes trials, ODYSSEY Outcomes with alirocumab, reported top-line results.5 This trial was conducted in a higher risk population, namely patients with a recent (1-12 months, median 2.6 months) acute coronary syndrome (ACS). ODYSSEY Outcomes has strengthened the evidence-base for PCSK9 inhibition and helped to dispel ongoing concerns about the safety of this therapeutic strategy.
What did ODYSSEY Outcomes show?
ODYSSEY Outcomes randomized 18,924 patients (mean age 58 years, 25% female, 19% with a prior MI, 48% with a NSTEMI) from 57 countries in North and Latin America, Europe, Asia, Australasia, Israel and South Africa; 9,462 patients each received alirocumab or placebo every 2 weeks. Beyond the clinical setting, there were three other major key inclusion criteria: aged at least 40 years; on high-intensity statin therapy (atorvastatin 40-80 mg daily, rosuvastatin 20-40 mg/daily, or maximum tolerated dose of these statins); and baseline LDL-C levels ≥70 mg/dl (1.8 mmol/L). Given concerns about the safety of very low LDL-C levels in ACS patients at the time of study inception, a titration strategy (75 or 150 mg alirocumab, or blinded placebo every 2 weeks) was used to maintain LDL-C levels within the target range of 25-50 mg/dL (0.65-1.29 mmol/L), although LDL-C levels as low as 15 mg/dL (0.39 mmol/L) were also considered acceptable. Overall, 7.7% of patients in the alirocumab group required a blinded switch to placebo as LDL-C levels were less than 15 mg/dL on active treatment.
The study population was very well treated with best evidence-based treatment that included high-intensity statin therapy (89%), aspirin, antithrombotics, angiotensin converting enzyme inhibitors/angiotensin receptor blockers and beta-blockers. At baseline, median LDL-C was 87 mg/dL (2.25 mmol/L), slightly lower than that observed in in FOURIER (92 mg/dL or 2.38 mmol/L).
Efficacy
The primary study endpoint was a composite of coronary heart disease (CHD) death, nonfatal MI, ischaemic stroke or unstable angina requiring hospitalization. The study predicted a 15% reduction in this primary endpoint (assuming a placebo event rate of 11.4% over 48 months). Thus, ODYSSEY Outcomes achieved its primary objective; the large reduction in LDL-C levels with alirocumab (61% at 12 months and 55% at 48 months) was associated with a 15% reduction (from 11.1% to 9.5%) in the primary endpoint (p=0.0003) over a median follow-up of 2.8 years (interquartile range 2.3-3.4 years) (Table 1). Using a hierarchical testing procedure, there were also statistically significant reductions in most of the main secondary outcomes, notably CHD events (a composite of CHD death, nonfatal MI, unstable angina requiring hospitalization, ischaemia-driven coronary revascularization), major CHD events (CHD death or nonfatal MI) and cardiovascular events. There was also a decrease in all-cause death (by 15%) but the p-value was regarded as nominal as the two prior endpoints (CHD death and cardiovascular death) were not statistically significant.
Table 1. Effect of alirocumab treatment on the primary efficacy outcome and its components
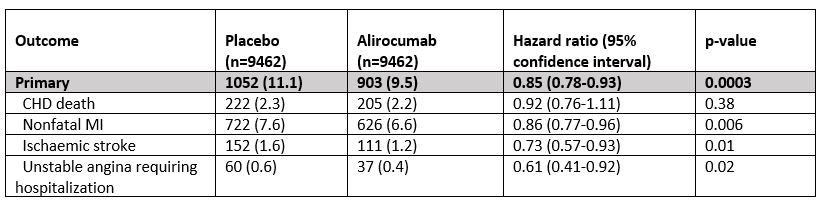
*Of the pre-specified subgroup analyses, only baseline LDL-C showed marginal interaction (p-value for interaction 0.09). In post hoc analyses, patients with a baseline LDL-C ≥100 mg/dl (2.6 mmol/L) derived pronounced clinical benefit from alirocumab treatment with a 24% reduction in the primary endpoint (Hazard ratio 0.76, 95% confidence interval 0.65-0.87) and 29% reduction in all-cause death (Hazard ratio 0.71, 95% confidence interval 0.56-0.90).
Safety
Alirocumab treatment was safe and well tolerated in this very high-risk patient population, with an adverse event profile consistent with that previously reported.6 The prevalence of injection site reactions was 1.7% higher than with placebo (3.8% versus 2.1%), and there was no signal for any increase in worsening diabetes or diabetes complications (18.8% versus 21.2% with placebo), new-onset diabetes (9.6% versus 10.1%), neurocognitive effects (1.5% versus 1.8%) or haemorrhagic stroke (<0.1% versus 0.2%).
ODYSSEY Outcomes and FOURIER: weighing the evidence
There are differences between ODYSSEY Outcomes and FOURIER, in terms of study design, results and duration of treatment. First, the study populations differ: ODYSSEY Outcomes was in an ACS setting whereas FOURIER was conducted in stable ASCVD patients, of whom 81% had a prior MI (median time from most recent MI, 3.3 years). Not surprisingly, therefore, there was a higher prevalence of high-intensity statin use in ODYSSEY Outcomes than in FOURIER (89% versus 69%). Moreover, the majority of patients (>60%) in ODYSSEY Outcomes were statin-naïve, in line with other studies in ACS patients,7-9 although full publication of the study is awaited to confirm these data.
The primary endpoint also differed between the two studies. FOURIER had a 5-point composite endpoint of cardiovascular death, MI, stroke, hospitalization for unstable angina and coronary revascularization, whereas in ODYSSEY Outcomes the primary endpoint was a 4-point composite mainly focused on coronary events, together with ischaemic stroke.
In terms of results, both studies achieved a consistent 15% reduction in the primary endpoint, although the impact of PCSK9 inhibition on the individual components of this endpoint differed. Reduction in MI was greater in FOURIER than in ODYSSEY Outcomes (27% versus 14%), whereas ODYSSEY Outcomes showed greater benefit on reduction in unstable angina (39% versus 1% in FOURIER). ODYSSEY Outcomes also showed a trend for benefit on cardiovascular and CHD death (reduction by 8-12%), as well as a decrease in all-cause mortality (by 15%).
Finally, ODYSSEY Outcomes has provided reassuring safety data, which when combined with findings from FOURIER, reaffirm the safety and tolerability of very low LDL-C levels attained with PCSK9 inhibition in high-risk patients.
Clinical implications
The results from both studies reinforce clinical guidance for the use of PCSK9 inhibition from a recent European Society of Cardiology/European Atherosclerosis Society Task Force.10 The pronounced clinical benefit observed in ODYSSEY Outcomes in patients with a higher baseline LDL-C value (≥100 mg/dL or 2.6 mmol/L) are consistent with the concept that absolute risk reduction is greater at higher starting levels of LDL -C.
The combined evidence from these two multinational cardiovascular outcomes studies also confirms that attainment of lower LDL-C levels, beyond current guideline-recommended goals, provides further reduction in cardiovascular risk. Thus, the question facing guideline groups is what should be the LDL-C goal in high risk patients? Considerations on this question, however, are complicated by the use of a titration scheme in ODYSSEY Outcomes, which aimed to maintain LDL-C levels within a target range. There is no doubt that lower is better – and safe- but the question is just how low to go.
What are the outstanding questions that remain about PCSK9 inhibition? Further evaluation of the characteristics of the subgroup in ODYSSEY Outcomes with baseline LDL-C levels ≥100 mg/dL on high intensity statin treatment is needed. Was there a higher prevalence of statin-naïve patients, who as a group may benefit more from PCSK9 inhibition? Is the benefit on mortality explained by stabilization of plaque composition and/or vulnerability with PCSK9 inhibition plus statin? Such a proposal would align with findings from the Myocardial Ischemia Reduction with Acute Cholesterol Lowering (MIRACL) trial, in which early intensive lowering of LDL-C levels with atorvastatin initiated 24-96 hours after an ACS reduced the primary endpoint, a composite of death, MI, resuscitated cardiac arrest, or worsening angina with new objective evidence of myocardial ischemia requiring urgent rehospitalization, at 4 months. Reduction in recurrent symptomatic ischaemia requiring rehospitalization was a key driver of this benefit.11
Ultimately, the uptake of PCSK9 inhibition is governed by payers. Will the possibility of reducing not only cardiovascular events but also death after an ACS tip the incremental cost-effectiveness ratio in favour of PCSK9 inhibition? We await the full publication of the ODYSSEY Outcomes trial, not only to fully evaluate this study, but also to discern answers to these urgent questions.
References
- Sabatine MS, Giugliano RP, Keech AC et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713-1722.
- Bonaca MP, Nault P, Giugliano RP et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation 2018;137:338-350.
- Sabatine MS, De Ferrari GM, Giugliano RP et al. Clinical benefit of evolocumab in patients with a history of MI: an analysis from FOURIER. American Heart Association Scientific Sessions, Late-breaking presentation.
- Ference BA, Cannon CP, Landmesser U et al. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J 2017; doi: 10.1093/eurheartj/ehx450. [Epub ahead of print]
- Schwartz GG, Szarek M, Bhatt DL et al. The ODYSSEY Outcomes Trial: topline results. Alirocumab in patients after acute coronary syndrome. Clinical Latebreaker, 67th Scientific Sessions of the American College of Cardiology, March 10th, 2018.
- Jones PH, Bays HE, Chaudhari U et al. Safety of alirocumab (A PCSK9 Monoclonal Antibody) from 14 Randomized Trials. Am J Cardiol 2016;118:1805-1811.
- Cannon CP, Braunwald E, McCabe CH et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504.
- Cannon CP, Blazing MA, Giugliano RP et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387-2397.
- Schwartz GG, Olsson AG, Abt M et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089-2099.
- Landmesser U, Chapman MJ, Stock JK et al. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J 2017; doi: 10.1093/eurheartj/ehx549. [Epub ahead of print]
- Schwartz GG, Olsson AG, Ezekowitz MD et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285:1711-1718.