The European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) Task Force has published updated clinical guidance for the use of PCSK9 inhibitors. This document supersedes the previous guidance issued by this group.1 The document is available to download here.
Landmesser U, Chapman MJ, Stock JK, Amarenco P, Belch JJF, Borén J, Farnier M, Ference BA, Gielen S, Graham I, Grobbee DE, Hovingh GK, Lüscher TF, Piepoli MF, Ray KK, Stroes ES, Wiklund O, Windecker S, Zamorano JL, Pinto F, Tokgözoğlu L, Bax JJ, Catapano AL. 2017 Update of ESC/EAS Task Force on Practical Clinical Guidance for PCSK9 inhibition in patients with ASCVD or in familial hypercholesterolaemia. European Heart Journal 2017; https://doi.org/10.1093/eurheartj/ehx549
FOURIER and SPIRE provide answers to previous uncertainties about PCSK9 inhibition
A key driver for this update has been the publication of the first cardiovascular outcomes trials with PCSK9 monoclonal antibody therapy. The FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial in 27,564 patients with clinical atherosclerotic cardiovascular disease (ASCVD) is the first completed outcomes study with these novel treatments. As previously reported,2 lowering of low-density lipoprotein cholesterol (LDL-C) levels by 59% (from a baseline of 2.4 mmol/L or ~90 mg/dL) with the addition of the PCSK9 monoclonal antibody evolocumab to background moderate to high intensity statin therapy significantly reduced the risk of major cardiovascular events (absolute event rates 9.8% versus 11.3% on placebo over 2.2 years, relative risk reduction of 15%). Most of this benefit was driven by reduction in nonfatal myocardial infarction (MI) and coronary revascularization. There were also important insights from the SPIRE (Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects) outcomes studies, despite the termination of bococizumab due to immunogenicity issues resulting in wide variability in LDL-C lowering response.3,4 These trials also included high risk patients with clinically diagnosed familial hypercholesterolaemia. In SPIRE-2, with a mean baseline LDL-C level of 3.4 mmol/L (133 mg/dL), there was a significant reduction in major cardiovascular events by 21% (absolute event rates 3.32% versus 4.19% on placebo, p=0.02) within 12 months. SPIRE-1, which had baseline LDL-C levels comparable to FOURIER, did not show any significant difference as the duration of treatment was short (7 months).3
A number of questions arise from consideration of these data. First, is the clinical benefit per mmol/L LDL-C reduction with a PCSK9 inhibitor consistent with that observed with statin treatment?
Initial comparison with the Cholesterol Treatment Trialists’ (CTT) Collaboration regression line, based on average response over 5 years treatment, indicated that the benefit was less in FOURIER.5 It is, however, recognized that the efficacy of statin therapy is less in the first year than in subsequent years.6 Thus, when the data were adjusted for duration of therapy, the results from FOURIER were superimposable with those observed with statin therapy (Figure 1).
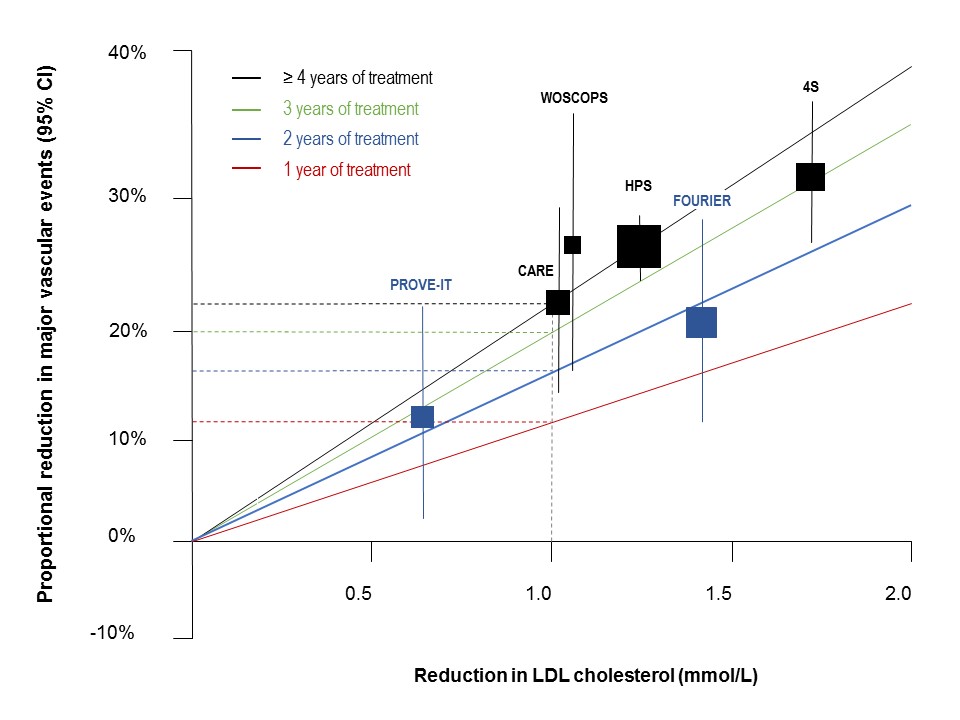
Second, is there a lower threshold for benefit from LDL-C lowering?
A recent report from FOURIER provides insights.7 Outcomes data were analyzed by LDL-C quintile at 4 weeks in 25,982 patients who did not experience a primary efficacy or pre-specified safety event before this time. There was a strong linear relationship between attained LDL-C level at 4 weeks and reduction in major cardiovascular events, with no evidence for a lower limit for cardiovascular benefit.
Third, how can we explain the lack of a mortality benefit in FOURIER?
It may be that the MI and strokes prevented were not serious enough, or followed long enough, to be reflected in reduced mortality. Indeed, the average duration of follow-up in FOURIER was 26 months, at which time the study had accrued the required number of secondary endpoints (a composite of cardiovascular death, MI or stroke) as specified in the statistical analysis.8 Alternatively, this result could be possibly explained by a play of chance, with some reassurance from SPIRE-2, which showed trends in favour of PCSK9 inhibition for the endpoints of cardiovascular and all-cause mortality.3 It is also important to bear in mind that reduction in mortality with statin therapy has only been observed after prolonged treatment, and not with a background of intensive LDL-C lowering therapy as in FOURIER.
So, in summary what can we learn from these trials regarding the clinical use of PCSK9 monoclonal antibody therapy?
- In very high-risk patients, the addition of a PCSK9 inhibitor to statin reduces LDL-C levels and this translates to reduction in cardiovascular events.
- Nonfatal MI is the key driver of this benefit.
- There is no evidence for a lower LDL-C threshold for cardiovascular benefit.
- PCSK9 inhibition appears to be safe and well tolerated over the duration of trials, although long-term surveillance is clearly needed.
Which patient groups should be targeted?
Translation of this evidence to clinical practice also needs to consider the cost of these novel therapies. Consequently, the Task Force prioritized two main patient groups for judicious use of PCSK9 inhibitor treatment (see Box 1).
| Box 1 Patient groups prioritized for PCSK9 inhibitor treatment |
| • Patients with clinical ASCVD and substantially elevated LDL-C levels. Patients should be on maximally tolerated statin therapy (ideally with concomitant ezetimibe, although this depends on local guidance). ASCVD patients unable to tolerate three or more statins at recommended doses are another priority group. • Familial hypercholesterolaemia (FH) patients without clinical ASCVD but with substantially elevated LDL-C levels despite treatment with maximally tolerated statin plus ezetimibe. |
What are the LDL-C thresholds to consider a PCSK9 inhibitor treatment?
Selection of the LDL-C levels (i.e. thresholds) for considering a PCSK9 inhibitor takes account of the baseline LDL-C level (on statin with or without ezetimibe) and the presence of additional indicators of risk severity. Three LDL-C thresholds are defined, with reduction by 50% (with a PCSK9 inhibitor) offering the possibility of attainment of guideline-recommended LDL C goal (see Box 2). In the case of ASCVD patients, the Task Force advises that imaging may help to identify those patients with severe and/or extensive disease at particularly high risk; the reader is referred to the text for direction.
As in the previous Task Force guidance, evolocumab is recommended as add-on LDL-C lowering therapy in patients with homozygous FH, who have residual LDL receptor activity (i.e. excluding patients with LDLR negative/negative mutations).1
| Box 2 LDL-C thresholds for considering a PCSK9 inhibitor | |
| Patients with clinical ASCVD • On maximally tolerated statin (with or without ezetimibe*) or with statin intolerance**. • With additional indices of risk severity (see below) | LDL-C threshold >3.6 mmol/L (140 mg/dL) >2.6 mmol/L or 100 mg/dL |
| Indices of risk severity: Concomitant diabetes mellitus with target organ damage or with a major risk factor such as marked hypertension; lipoprotein(a) >50 mg/dL; major risk factors such as smoking, marked hypertension; >40 years without treatment; premature ASCVD (<55 years in males and <60 years in females) in first degree relatives; and imaging indicators of increased risk. These were based on the 6th Joint Task Forces guidelines for CVD prevention and the SAFEHEART Registry data.9,11 | |
| FH patients without clinical ASCVD • On maximally tolerated statin plus ezetimibe • With additional indices of risk severity (see below | LDL-C threshold >4.5 mmol/L (180 mg/dL) >3.6 mmol/L (140 mg/dL) |
| Indices of risk severity: Concomitant diabetes mellitus with target organ damage or with a major risk factor such as marked hypertension; lipoprotein(a) >50 mg/dL; major risk factors such as smoking, marked hypertension; >40 years without treatment; premature ASCVD (<55 years in males and <60 years in females) in first degree relatives; and imaging indicators of increased risk. These were based on the 6th Joint Task Forces guidelines for CVD prevention and the SAFEHEART Registry data.9,11 | |
| Footnotes * Add-on ezetimibe is recommended, although it is recognized that this may be insufficient in very high-risk patients requiring more than 50% LDL-C reduction to attain goal. ** unable to tolerate three or more statins at recommended doses, as defined by the EAS Consensus Panel statement on statin-associated muscle symptoms12 | |
How to monitor the LDL-C response?
An important aspect to this new guidance is information on monitoring the LDL-C lowering response to PCSK9 inhibitor therapy. The guidance re-emphasizes the need to check adherence as a potential cause of failure to attain LDL-C goal with statin therapy, before considering non-statin options. In those patients with LDL-C levels above the thresholds specified in Box 2, a PCSK9 inhibitor can be considered. The Task Force recommends assessment of the LDL-C lowering response at 2 weeks after first injection (before the next injection if using a 2-weekly regimen for PCSK9 inhibition).
The Task Force recognizes, however, that (beyond bococizumab, now terminated) there is very limited information about interindividual variability in the LDL-C lowering response to a PCSK9 inhibitor. There are initial insights from registry data from a cohort of FH patients. In 79 heterozygous FH patients, the median LDL-C reduction with alirocumab or evolocumab was 58%, although the interquartile range varied from 43% to 72%; 10 patients had <30% LDL-C reduction.13 Clearly more information is needed about the extent of variability in response to PCSK9 monoclonal antibody therapy in routine clinical use.
What are the remaining gaps in knowledge about PCSK9 inhibition?
To date, low levels of LDL-C attained on a PCSK9 inhibitor appear to be safe within the relatively limited duration of the clinical trials. Information about the long-term safety of these novel therapies is needed from the extended use of these agents in clinical practice. One key question relates to the risk for new-onset diabetes, as has been suggested by Mendelian randomization studies.14,15 This is especially relevant in individuals with the metabolic syndrome cluster or with prediabetes. Although analyses of trial data suggest no increase in risk, even at very low LDL-C levels,16-18 it should be borne in mind that the modest risk for incident diabetes recognized with statin therapy (~1 per 1000 patient-years exposure) was only established after long-term follow-up.19
Another potential concern for clinicians has been the possibility of adverse effects on cognitive function with the very low LDL-C levels attained on a PCSK9 inhibitor. EBBINGHAUS (Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects), a nested cohort study of the FOURIER trial, specifically addressed this issue using a validated battery of neurocognitive tests (Cambridge Neuropsychological Test Automated Battery [CANTAB] Assessment). While there was no evidence of significant differences in cognitive function in either evolocumab or placebo groups in EBBINGHAUS, the patient population in EBBINGHAUS may have been less than ideal (mean age 63 years) and the duration of treatment was short (19 months).20
It is also important to bear in mind that the FOURIER and SPIRE trials enrolled patients with stable ASCVD. To date, we lack information regarding PCSK9 inhibition in patients with acute coronary syndromes (ACS), who are known to be at high risk of recurrent events. Does this patient population have characteristics that may favour or negatively impact the efficacy of PCSK9 inhibition? The ODYSSEY Outcomes trial which is enrolling patients with a recent ACS (<3 months from the time of the index event to randomization) will offer important insights about the potential of PCSK9 inhibition in this very high risk patient group (Box 3).
| Box 3 Comparison FOURIER and ODYSSEY Outcomes, patient baseline data | ||
| FOURIER2 | ODYSSEY OUTCOMES22 | |
| Clinical setting | Stable ASCVD; median of 3.4 years from most recent MI | Recent ACS; median 2.6 months from index event |
| Statin use | • 99.7% on moderate to high intensity statin | • 67% were statin-naïve >3 months before index event • 89% were on high-intensity statin at randomization |
| Ezetimibe at randomization | 5.3% | 3% |
| Cardiovascular history* | • 81% had a history of MI, 20% had previous nonhaemorrhagic stroke and 13% had peripheral arterial disease | • <50% had a history of MI and/or coronary revascularization |
| Baseline LDL-C | 2.37 (2.07-2.79) mmol/L 92 (80-108) mg/dL | 2.25 (1.89-2.69) mmoL/L 87 (73-104) mg/dL |
| * at baseline | ||
Other gaps in knowledge about PCSK9 inhibition relate to the use of PCSK9 inhibition in other very high risk groups that to date have not been studied in clinical trials, specifically patients with chronic kidney disease (not requiring dialysis).9 Finally, the cost-effectiveness of this therapy added to maximally tolerated statin, ideally with concomitant ezetimibe therapy, is a highly pertinent concern with the finite budgets of healthcare systems.
In the space of little more than a decade, clinicians now have at their disposal a novel therapy that substantially lowers LDL-C levels when added to a statin, and reduces major cardiovascular events in very high-risk patients with clinical ASCVD. Judicious use of this treatment, targeting those patients most likely to derive benefit, is needed. This new ESC/EAS Task Force statement provides important clinical guidance and practical algorithms that will help clinicians in their routine practice.
References
1. Landmesser U, Chapman MJ, Farnier M et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J 2017;38:2245-55.
2. Sabatine MS, Giugliano RP, Keech AC et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713-22.
3. Ridker PM, Revkin J, Amarenco P et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017;376:1527-39.
4. Ridker PM, Tardif JC, Amarenco P et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med 2017;376:1517-26.
5. Ference BA, Cannon CP, Landmesser U et al. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J. doi: 10.1093/eurheartj/ehx450. [Epub ahead of print]
6. Collins R, Reith C, Emberson J et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532-61.
7. Giugliano RP, Pedersen TR, Park JG et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 2017. doi: 10.1016/S0140-6736(17)32290-0. [Epub ahead of print]
8. Sabatine MS, Giugliano RP, Keech A et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J 2016;173:94-101.
9. Piepoli MF, Hoes AW, Agewall S et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315-81.
10. Suárez C, Zeymer U, Limbourg T et al. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc Med 2010;15:259-65.
11. Pérez de Isla L, Alonso R, Mata N et al. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART Registry. Circulation 2017;135:2133-44.
12. Stroes ES, Thompson PD, Corsini A et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012-22.
13. Galema-Boers AMH, Lenzen MJ, Sijbrands EJ, Roeters van Lennep JE. Proprotein convertase subtilisin/kexin 9 inhibition in patients with familial hypercholesterolemia: Initial clinical experience. J Clin Lipidol 2017;11:674-81.
14. Ference BA, Robinson JG, Brook RD et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016;375:2144-53.
15. Schmidt AF, Swerdlow DI, Holmes MV et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol 2017;5:97-105.
16. Colhoun HM, Ginsberg HN, Robinson JG et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J 2016;37):2981-9.
17. Sattar N, Toth PP, Blom DJ et al. Effect of the Proprotein Convertase Subtilisin/Kexin Type 9 inhibitor evolocumab on glycemia, body weight, and new-onset diabetes mellitus. Am J Cardiol 2017;120:1521-7.
18. Sabatine MS, Leiter LA, Wiviott SD et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017. doi: 10.1016/S2213-8587(17)30313-3. [Epub ahead of print].
19. Sattar N, Preiss D, Murray HM et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735-42.
20. Giugliano RP, Mach F, Zavitz K et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017;377:633-43.
21. Schwartz GG, Bessac L, Berdan LG et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J 2014;168:682-9.
22. Goodman SG, Schwartz GG, Bhatt DL et al. Use of high-intensity statin therapy post-acute coronary syndrome in the ongoing ODYSSEY OUTCOMES trial of alirocumab, a proprotein convertase subtilisin/kexin type 9 monoclonal antibody, versus placebo: interim baseline data. J Am Coll Cardiol 2017;69:11(Supplement):153 (abstract).