Statin –associated muscle symptoms :: Latest EAS Consensus Panel paper focuses on this neglected patient group
Statins are the cornerstone for lowering low-density lipoprotein cholesterol (LDL-C) for cardiovascular disease (CVD) prevention. Moreover, as reported recently, the benefits of statin therapy extend similarly to men and women.1 While statins are safe and well tolerated, like all treatments, a proportion of patients report side effects.
Muscle symptoms are the most prevalent side effects reported with statin therapy, and one of the main reasons for non-adherence or discontinuation of treatment. While it is recognised that statins do cause a rare side effect known as myositis, defined as muscle symptoms in association with a substantially elevated serum creatine kinase [CK] concentration, most statin-associated muscle symptoms (SAMS) are not accompanied by marked CK elevation. Until recently, the issue of SAMS has been largely neglected. Increasing calls to focus international attention on this important clinical problem prompted the European Atherosclerosis Society (EAS) Consensus Panel to address this contentious issue, and, importantly, review what is known to date about the underlying pathophysiology.
According to lead author, Professor Erik Stroes, Department of Vascular Medicine, Academic Medical Center, Amsterdam, the Netherlands: ‘While there has been tremendous progress in cardiovascular prevention using statin therapy, the persistently high cardiovascular risk in patients failing to adhere to statins due muscle symptoms has been overlooked. This EAS Consensus Paper has addressed this important unmet clinical need. The probability of SAMS being caused by statins is based on the nature of symptoms and their temporal relationship with statin initiation, discontinuation, and repetitive re-challenge. Optimal therapy for patients who have SAMS should combine a maximally tolerated, or even non-daily statin dose, together with non-statin-based lipid-lowering therapies in order to achieve LDL-C targets and optimise CVD benefit.’
The EAS Consensus Panel Statement is available to read at this link [http://eurheartj.oxfordjournals.org/content/early/2015/02/18/eurheartj.ehv043].
How common are SAMS?
Data from patient registries, as well as clinical experience, indicate that 7-29% of patients complain of SAMS.2-6 In contrast, the proportion of patients reporting muscle symptoms in blinded randomised controlled trials has been lower, with myalgia rates similar in those on statin or placebo.7-9
As far as the Panel is aware, the Effects of Statins on Muscle Performance (STOMP) study10 is the only randomised, double-blind, placebo-controlled study that was specifically designed to examine the effect of statins on skeletal muscle symptoms and performance. Among the 420 statin-naïve subjects randomised to atorvastatin 80 mg daily or placebo for 6 months, 9.4% of the statin-treated and 4.6% of control subjects met the study definition of myalgia (p=0.054). Yet even with this lower incidence of muscle complaints (compared with observational studies), a substantial number of patients will experience SAMS, given the widespread use of statins.
What is their typical presentation?
SAMS are typically characterised by muscle pain, weakness and aches, usually symmetrical and proximal, generally affecting large muscle groups including the thighs, buttocks, calves and back muscles. While these tend to occur early (within 4¬6 weeks after starting a statin10), SAMS have been reported after many years of treatment. Symptoms may occur with an increase in statin dose or initiation of an interacting drug, and are often more common in physically active individuals.2 SAMS often appear more promptly when patients are re-challenged with the same statin.
In most patients, SAMS are not accompanied by marked CK elevation.11,12
Which patients are at risk?
Risk factors for SAMS are summarised in Table 1.13 Other factors that increase statin blood levels can increase the likelihood of developing SAMS.14 These may include the use of high-dose statin therapy, polypharmacy, and drug-drug interactions, such as those involving gemfibrozil, macrolides, azole antifungal agents, protease inhibitors and immunosuppressive drugs), as well as inhibitors of cytochrome P450 isoenzymes, organic anion transport protein 1B1 [OATP 1B1], or P-glycoprotein 1 [P-gp].15
Note that the presence of an increasing number of factors is associated with greater risk for SAMS.14 16
| Table 1. Risk factors for SAMS |
| • Patient factors: very elderly (>80 years), female, low body mass index, Asian descent • Other predisposing factors: History of CK elevation or unexplained muscle/joint/tendon pain, inflammatory or inherited metabolic, neuromuscular/muscle defects, previous statin-induced myotoxicity, myopathy on other lipid-lowering therapy • Diet/lifestyle: excessive physical activity, overconsumption of grapefruit or cranberry juice, alcohol or drug abuse • Concurrent conditions: acute infection, impaired renal or hepatic function, diabetes, HIV (both the condition and HIV medications such as protease inhibitors), vitamin D deficiency, organ transplant recipients, severe trauma, biliary tree obstruction • Surgery with high metabolic demands • Genetic factors (see below) |
How can clinicians best diagnose SAMS?
Definitive diagnosis of SAMS is difficult because symptoms are subjective and there is no “gold standard” diagnostic test and no validated muscle symptom questionnaire.
The EAS Consensus Panel proposes a clinical definition for the probability of SAMS, based on the nature of the muscle symptoms (i.e. muscle pain or aching), and their temporal association with statin initiation, discontinuation, and response to repetitive statin re-challenge.
How can clinicians best manage SAMS?
If a patient complains of muscle symptoms, the EAS Consensus Panel proposes the following initial course of action:
- Evaluate risk factors which can predispose to statin-associated myopathy (see Table 1)
- Exclude secondary causes (especially hypothyroidism and other common myopathies such as polymyalgia rheumatica, or increased physical activity) (see Table 1)
- Consider concomitant medication: other commonly prescribed drugs may also cause muscle-related side effects; consider also drug-drug interactions that may increase statin blood levels and risk for SAMS (see Table 1)
- Review the indication for statin use.
Management of SAMS depends on the level of CK elevation, and the patient’s CVD risk (see Table 2). Note that most patients who complain of muscle symptoms have normal or mild to moderately elevated CK levels (<4 X ULN).17
| Table 2. Management strategies for SAMS | |
| Patients with muscle symptoms and CK < 4 X ULN | |
| At low CVD risk | • Consider the benefits of therapeutic lifestyle changes versus risk of continuing the statin |
| At high CVD risk | • Consider the benefits of ongoing statin therapy versus the burden of muscle symptoms • Withdrawal of statin therapy followed by one or more re-challenges (after a washout) may help in determining causality • Consider an alternative statin, a statin at lowest dose, intermittent (i.e. non-daily) dosing of a highly efficacious statin, or the use of other lipid lowering medications |
| Patients with muscle symptoms and CK > 4 X ULN | |
| In patients at high CVD risk | • If CK is < 10 X ULN; continue the statin while monitoring CK. • If CK is > 10 X ULN and there is no secondary cause, stop the statin.If CK levels subsequently decrease, consider re-starting statin at a lower dose, or start a lower dose of an alternative statin, while monitoring symptoms and CK. • If CK elevation persists, consider referral to a neuromuscular specialist for investigation of an underlying myopathy. • If rhabdomyolysis is suspected, do not re-start statin. These patients, and those with very high CK levels (e.g. > 40 X ULN), should be referred for assessment of renal damage. Non-statin LDL-lowering therapy may be considered. |
What are the therapeutic options?
Treatment options include both statin and non-statin based therapy, as summarised in Table 3 and Figure 1.
| Table 3. Treatment options in patients with SAMS | |
| Statin | Wherever possible, the clinician should aim to maintain statin treatment given the associated CVD benefit. If symptoms/CK abnormalities resolve after discontinuation of statin, consider: • The same statin at a lower dose. If this lower dose is tolerated, consider uptitration so as to achieve as much LDL-C reduction with minimal muscle complaints. • An alternative statin, starting at a lower dose and if tolerated, uptitrating to optimise LDL-C reduction. If patients do not tolerate these strategies, consider alternate day or twice-weekly dosing: Preferably with a high intensity statin with a long half-life (atorvastatin, rosuvastatin and pitavastatin) Studies have shown that ∼70% of previously intolerant patients are able to tolerate these dosing strategies.18 |
| Non-statin First choice | • Ezetimibe should be considered the first choice, based on its safety profile, as well as recent evidence of CVD outcomes benefit in IMPROVE-IT.19,20 |
| Secondary choices | • Additional approaches, after ezetimibe, are bile acid sequestrants or fibrates in combination with ezetimibe, as needed to achieve LDL-C lowering consistent with guidelines |
| Nutraceuticals | • In addition to a low saturated fat diet and avoidance of trans fats, consider viscous fibre (mainly psyllium, 10 g daily) and foods with added plant sterols or stanols, either alone or with pharmacotherapy, depending on CVD risk. |
| Complementary therapies | Red yeast rice: • This is a fermented product, containing monacolin K, a product similar to lovastatin, and plant sterols. • Although recent data indicate that red yeast rice lowers LDL-C and is well tolerated, efficacy and safety in the long-term have yet to be established.21 There is also lack of standardisation between preparations, as well as the possibility of SAMS due to the statin-like content. Other therapies: • Ubiquinone (coenzyme Q10) and vitamin D supplementation have been suggested. • On current evidence, the Panel does not recommend these products in the management of patients with SAMS. 22-24In addition to a low saturated fat diet and avoidance of trans fats, consider viscous fibre (mainly psyllium, 10 g daily) and foods with added plant sterols or stanols, either alone or with pharmacotherapy, depending on CVD risk. |
Novel treatments, PCSK9 inhibitors and cholesteryl ester transfer protein (CETP) inhibitors, may offer future potential.
Figure 1.
EAS Consensus Panel algorithm for management of SAMS
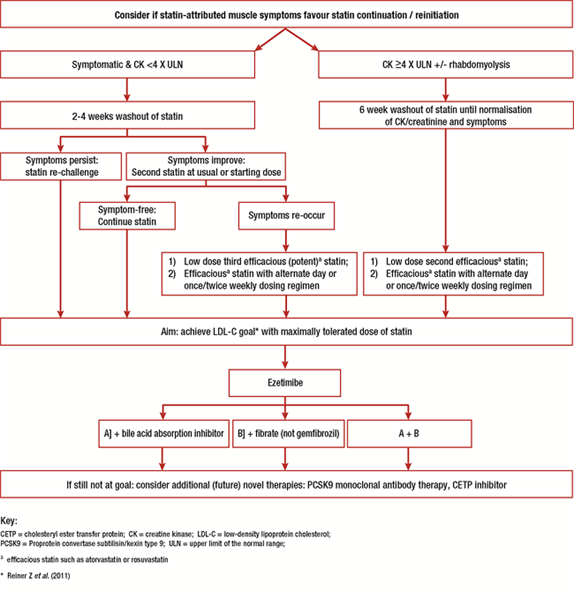
1 Efficacious statin such as atorvastatin, rosuvastatin or pitavastatin
* LDL-C goal in accordance with current guideline recommendations
What insights do we have into the underlying pathophysiology of statin myopathy?
A number of mechanisms have been proposed (see Figure 2).25 These include:
- reduced levels of non-cholesterol end-products of the mevalonate pathway
- reduced sarcolemmal and/or sarcoplasmic reticular cholesterol
- increased myocellular fat and/or sterols
- inhibition of production of prenylated proteins or guanosine triphosphate (GTP)ases
- alterations in muscle protein catabolism; decreased myocellular creatine
- changes in calcium homeostasis
- immune-mediated effects of statins. Studies suggest that statins may trigger the development of anti-HMG-CoA-reductase antibodies, dependent upon exposure, male gender, diabetes and genetic background.26.
- effects on mitochondrial function.
Figure 2.
Effects potentially involved in statin-related muscle injury/symptoms.25
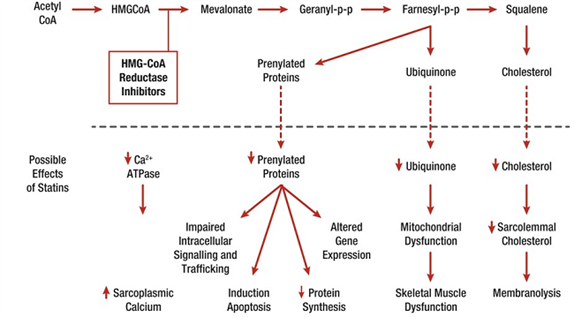
The interaction of statins with muscle mitochondria has been a key focus of interest. Abnormal mitochondrial function with depletion of ubiquinone (coenzyme Q10) has been reported in individuals on statin therapy, even if they are asymptomatic. Available evidence suggests that statins decrease mitochondrial function, attenuate energy production, and alter muscle protein degradation, each of which may contribute to the onset of muscle symptoms.27 Additionally, in a preclinical model, very recent data indicate that co-administration of a statin may aggravate myopathy/myositis associated with lipin-1 deficiency in mice, and that both statin and lipin-1 deficiency reduced autolysosome maturation.28
Is genetic testing a viable option in the future?
There are some clear genetic signals. Some variants of genes encoding drug transporters in both the liver and skeletal muscle, (most notably SLCO1B1, encoding OATP1B1), that increase serum statin concentration have been linked to muscle side effects.29 Other candidate genes for statin-induced myalgia have been identified, including genotypes associated with statin-induced down regulation of expression of the gene encoding glycine amidinotransferase (GATM).30 GATM catalyses a critical step in hepatic and renal synthesis of creatine, used in muscle to form creatine phosphate, which is a major source of energy storage in muscle. However, findings from studies have been controversial and further investigation is clearly needed.31,32
Finally, while identifying underlying genetic risk factors implicated in statin myopathy may have future potential, current evidence does not support recommendations for genetic testing as part of the diagnostic work-up of patients with SAMS.
In conclusion, this EAS Consensus Panel statement provides important clinical guidance for diagnosis and management of SAMS. Uniquely, this statement also offers a novel overview of potential mechanisms implicated in statin myopathy. Ultimately, improved recognition of clinically relevant SAMS and the use of a structured management work-up will help clinicians optimise CVD risk in their patients with SAMS.
References
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet Published Online January 9, 2015.
- Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovasc Drugs Ther 2005;19:403-14.
- Buettner C, Rippberger MJ, Smith JK et al. Statin use and musculoskeletal pain among adults with and without arthritis. Am J Med 2012;125:176-82.
- Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208-15.
- Zhang H, Plutzky J, Skentzos S et al. Discontinuation of statins in routine care settings: a cohort study. Ann Int Med 2013;158:526-34.
- El-Salem K, Ababeneh B, Rudnicki S et al. Prevalence and risk factors of muscle complications secondary to statins. Muscle Nerve 2011;44:877-81.
- Kashani A, Phillips CO, Foody JM et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 2006;114:2788-97.
- Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J 2014;168:6-15.
- Finegold, JA, Manisty CH, Goldacre, B, BarronAJ, Francis DP. What proportion of symptomatic side effects in patients taking statins are genuinely caused by the drug? Systematic review of randomized placebo-controlled trials to aid individual patient choice. Eur J Prev Cardiol 2014; 21:464-76.
- Parker BA, Capizzi JA, Grimaldi AS et al. Effect of statins on skeletal muscle function. Circulation 2013;127:96-103.
- MRC/BHF Heart Protection Study Collaborative Group. Effects of simvastatin 40 mg daily on muscle and liver adverse effects in a 5-year randomized placebo-controlled trial in 20,536 high-risk people. BMC Clinical Pharmacology 2009;9:6.
- Downs JR, Clearfield M, Tyroler HA et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Additional perspectives on tolerability of long-term treatment with lovastatin. Am J Cardiol 2001;87:1074-9.
- Mancini GB, Tashakkor AY, Baker S et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol 2013;29:1553-68.
- Armitage J. The safety of statins in clinical practice. Lancet 2007;370:1781-90.
- Corsini A. The safety of HMG-CoA reductase inhibitors in special populations at high cardiovascular risk. Cardiovasc Drugs Ther 2003;17:265-85.
- Ahmad Z. Statin intolerance. Am J Cardiol 2014;113:1765-71.
- Alfirevic A, Neely D, Armitage J et al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther 2014;96:470-6.
- Keating AJ, Campbell KB, Guyton JR. Intermittent nondaily dosing strategies in patients with previous statin-induced myopathy. Ann Pharmacother 2013;47:398-404.
- Norata GD, Ballantyne CM, Catapano AL. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur Heart J 2013;34:1783-9.
- Cannon CP, IMPROVE-IT Investigators. IMProved Reduction of Outcomes: Vytorin Efficacy International Trial. A multicenter, double-blind, randomized study to establish the clinical benefit and safety of Vytorin (ezetimibe/simvastatin tablet) vs simvastatin monotherapy in high-risk subjects presenting with acute coronary syndrome. http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_469669.pdf.
- Li Y, Jiang L, Jia Z et al. A meta-analysis of red yeast rice: an effective and relatively safe alternative approach for dyslipidemia. PLoS One 2014;9:e98611.
- Taylor BA, Lorson L, White CM, Thompson PD. A randomized trial of Coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis 2015;238:329-35.
- Banach M, Serban C, Sahebkar A et al, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Effects of Coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clinic Proc 2015;90:24-34.
- Michalska-Kasiczak M, Sahebkar A, Mikhailidis DP et al; Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Analysis of vitamin D levels in patients with and without statin-associated myalgia – a systematic review and meta-analysis of 7 studies with 2420 patients. Int J Cardiol 2014;178C:111-6.
- Needham M, Mastaglia FL. Statin myotoxicity: a review of genetic susceptibility factors. Neuromuscul Disord 2014;24:4-15.
- Mikus CR, Boyle LJ, Borengasser SJ et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol 2013;62:709-14.
- Limaye V, Bundell C, Hollingsworth P et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 2014 [Epub ahead of print].
- Zhang P, Verity MA, Reue K. Lipin-1 regulates autophagy clearance and intersects with statin drug effects in skeletal muscle. Cell Metab 2014;20:267-79.
- SEARCH Collaborative Group, Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy–a genome wide study. N Engl J Med 2008;359:789-9.
- Ruaño G, Windemuth A, Wu AH et al. Mechanisms of statin-induced myalgia assessed by physiogenomic associations. Atherosclerosis 2011;218:451-6.
- Mangravite LM, Engelhardt BE, Medina MW et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature 2013;502:377-80.
- Carr DF, Alfirevic A, Johnson R et al. GATM gene variants and statin myopathy risk. Nature 2014;513:E1.